One of the big issues with understanding how rapamycin works in healthy people trying to prevent aging related disease and disfunction is that virtually all the clinical studies done with rapamycin over the past 20+ years, have been done in very sick people. Specifically, people who have had organ transplants and are on a large number of drugs, or people who have cancer or heart disease and who again may be being treated with many other drugs simultaneously. In these applications the patients take rapamycin at high daily doses. So the side effects (and benefits) are going to be very different from what people will experience if they are healthy, taking low weekly doses of rapamycin as used in anti-aging.
Rapalog Enhances Flue Shot Effectiveness by 20%, Minor Side Effects on Weekly Dose
The most important paper that really kickstarted the use of rapamycin for anti-aging in humans and highlighted both the potential benefits, and minimal side effects when taken weekly, was the 2014 Mannick paper (shown below).
In this study they used a rapamycin clone (Everolimus/RAD001/Afinitor, a small molecule drug almost identical to rapamycin, but slightly different, and patented by Novartis) in a study in healthy elderly people to see if by using Everolimus on a weekly dose, they could rejuvenate the aging immune system to result in a better response to the flu vaccine. The trial was successful and with only minimal side effects as shown below.
“To begin to assess the effects of mTOR inhibition on human aging related conditions, we evaluated whether the mTOR inhibitor RAD001 ameliorated immunosenescence (the decline in immune function during aging) in elderly volunteers, as assessed by their response to influenza vaccination. RAD001 (Everolimus) enhanced the response to the influenza vaccine by about 20% at doses that were relatively well tolerated.”
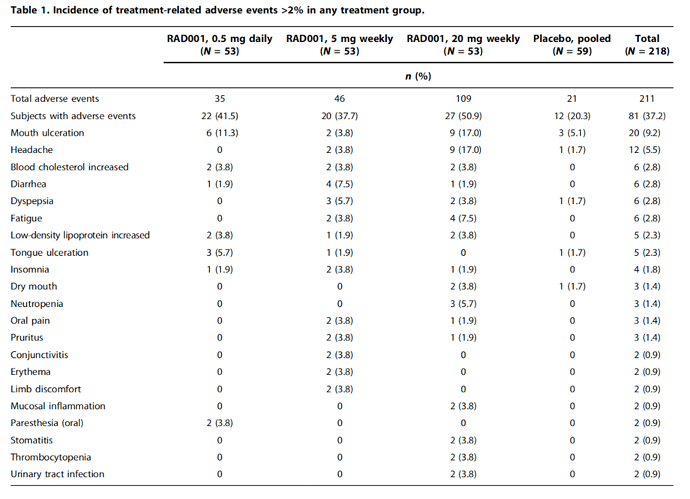
Side Effects of Weekly Everolimus (RAD001) in Healthy Adults
As can be seen below, the side effect profile for weekly use of a rapalog is quite benign. Mouth canker sores (mouth ulcerations), and headaches, are the most frequent issues, both of which go away without treatment quite quickly. The rest of the “side effects” are only approximately 1% higher in frequency than the placebo. The finding in this study correlate quite well with the experience seen in the rapamycin users as shown in this post on “Side Effects - personal experiences”.
mannick2014Everolimus.pdf (354.2 KB)
Here are some of the few studies that have been done on healthier people taking rapamycin:
Link to Sci-Hub Full Paper:
https://sci-hub.se/10.1097/00007691-200010000-00006
10.1097@00007691-200010000-00006-3.pdf (163.8 KB)