Could you discuss some hypothetical IV solution’s?
IV formulations for rapamycin? Oh dear, as if IM wasn’t hard enough…
That patent RapAdmin posted goes over one formulation, and they likely wouldn’t have applied for one if it didn’t work. Usually you will see some combination of a polyethylene glycol, buffering agent, or preservative, all of which are used in that patent.
The issue with IV is that you don’t want to disturb the balances in blood. Namely, pH, hypertonicity, and hemolysis. Here’s a good article from Cambridge about how IV formulations are designed.
The shelf life of IV formulas tends to be short - water is not a good long-term storage solution. Solutions have to be constantly remade for freshness. Plus, it would be very unpleasant to have to do IV injections all the time.
Like I mentioned before, an IV form of rapamycin would be for hospital settings, where a constant dose is fed through a line. I don’t think it would ever be practical, or even desirable, for our use-case.
It seems like anyone who is interested in this area should read up and learn about compounding pharmacy techniques and science, and also basics of pharmacokinetics such as is covered in these types of books:
Compounding Sterile Preparations, Author(s):E. Clyde Buchanan, Philip J. Schneider, Ryan A. Forrey|
The essential sterile compounding reference every pharmacist needs has been fully updated and is the most comprehensive and authoritative reference available on sterile compounding available
The Art, Science and Technology of Pharmaceutical Compounding, Author(s):Jr. Allen, Loyd
Key Features: The initial chapters describe the requisite facilities and equipment, record keeping, calculations, and quality control.
Three new chapters: “Pharmaceutical Compounding Errors,” “Foams,” and “Compounding with Special Ingredients.”
Fifteen chapters are devoted to the compounding of each dosage form in turn, from powders and granules to injectables.
Applied Pharmaceutics in Contemporary Compounding, Author(s):Robert P. Shrewsbury
Pharmaceutical Compounding and Dispensing, Second Edition, Author(s):John F., Ph.D. Marriott, Keith A, Ph.D. Wilson, Christopher A., Ph.D. Langley, Dawn Belcher
Drug Delivery Approaches: Perspectives from Pharmacokinetics and Pharmacodynamics,
Author(s):Bret Berner
Drug Delivery Approaches: Perspectives from Pharmacokinetics and Pharmacodynamics delivers a thorough discussion of drug delivery options to achieve target profiles and approaches as defined by physical and pharmacokinetic models. The book offers an overview of drug absorption and physiological models, chapters on oral delivery routes with a focus on both PBPK and multiple dosage form options. It also provides an explanation of the pharmacokinetics of the formulation of drugs delivered by systemic transdermal routes.
Drug Metabolism, Pharmacokinetics and Bioanalysis, Author(s):|Hye Suk Lee (editor), Kwang-Hyeon Liu (editor)
Drug metabolism/pharmacokinetics and drug interaction studies have been extensively carried out in order to secure the druggability and safety of new chemical entities throughout the development of new drugs. Recently, drug metabolism and transport by phase II drug metabolizing enzymes and drug transporters, respectively, as well as phase I drug metabolizing enzymes, have been studied. A combination of biochemical advances in the function and regulation of drug metabolizing enzymes and automated analytical technologies are revolutionizing drug metabolism research.
Introduction to Drug Disposition and Pharmacokinetics, Author(s):Stephen H. Curry, Robin Whelpton
The application of knowledge of drug disposition, and skills in pharmacokinetics, are crucial to the development of new drugs and to a better understanding of how to achieve maximum benefit from existing ones. The book takes the reader from basic concepts to a point where those who wish to will be able to perform pharmacokinetic calculations and be ready to read more advanced texts and research papers.
Essential Pharmacokinetics: A Primer for Pharmaceutical Scientists, Author(s):|Thorsteinn Loftsson
Essential Pharmacokinetics: A Primer for Pharmaceutical Scientists is an introduction to the concepts of pharmacokinetics intended for graduate students and new researchers working in the pharmaceutical sciences. This book describes the mathematics used in the mammillary model as well as the application of pharmacokinetics to pharmaceutical product development, and is useful as both a self-study and classroom resource. Content coverage includes detailed discussions of common models and important pharmacokinetic concepts such as biological half-life, clearance, excretion, multiple dosage regimens and more.
I notice that in this study at the University of Washington, they used injection rapamycin: Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice - PMC
that was compounded / formulated as follows:
For the injection experiments, rapamycin (LC Laboratories) was dissolved in DMSO to 100 mg/ml, then further diluted in 5% PEG-400/5% Tween-80 to a final concentration of 1.2 mg/ml, sterile filtered, and stored at −80°C for long-term storage. 37 rapamycin treated mice (17 males, 20 females) were i.p. injected daily for 3 months with 66 µl/10g body weight for a final dosage of 8.0 mg/kg starting at 20–21 months old. 38 Control mice (18 males, 20 females) were i.p. injected with vehicle solution (5% PEG-400/5% Tween-20) for 3 months.
@O_o are these commonly used excipients in injection formulations for animals and humans?
PEG-400 is indeed a common one. There is an entire family of polyethylene glycols (PEGs) that are used to help solubilize drugs. You might also see PEG-300. The number just indicates the molecular weight (MW). The higher MW PEGs are usually used for more difficult, larger drugs, which makes sense for rapamycin. It’s well-tolerated in medical applications, so it’s quite standard among many formulations.
Tween-80 (or polysorbate 80) is, as your source states, a nonionic surfactant (mild soap) that also helps solubilize difficult-to-dissolve drugs. However, because it is a soap, it can break apart membranes if used in a high enough concentration. This is an issue for red blood cells, which has a rather delicate membrane. As such, Tween-80 and other surfactants are hemolytic (bursts open red blood cells) at higher concentrations, thus why that formulation keeps it at 5%. Currently, Tween-80 is being phased out of a lot of formulations because of this.
Updated labs on 6 months of therapeutic dosing Intramuscular + Intranasal IM.
Just a reminder, I am taking at least 10X the weekly dose than what most everyone is taking orally. (1 week post dose trough sirolimus level/AUC as reference).
Based on above MK study update, looks like 99% of people are taking 10mg/week or less ORALLY, which would likely produce <1 ng/mL at 1 week trough.
For example, if your 1 week trough Sirolimus is 0.75 ng/mL, then my Jan 25, 23 trough of 7.5 ng/mL is 10X the dose. My 11.1 ng/ml trough Aug 3 would be almost 15X the typical daily oral dose.
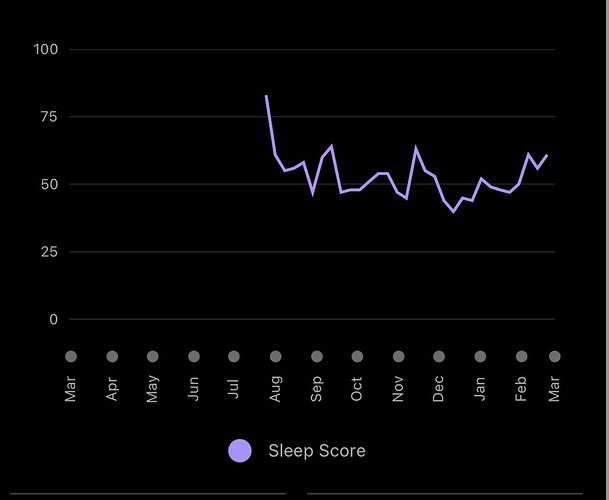
I am currently in washout (procedure, doc wanted all meds stopped, so out of abundance of caution), although feeling normal at 6 months, still able to do daily high exercise, even though full on anemic. However there seems to be a pretty strong negative sleep signal (Garmin watch sleep score). It’s definitely been trending downward on this protocol…more fragmented sleep, I feel more wired at night (take weekly dose in the AM). From summary below, I started high dose rapamycin in July 2022. There is a definite trend downward throughout the 6 month intervention (last dose Jan 18, 23)
At time of writing, my sleep score has been trending back up on washout (per above summary), although I have added a confounder, since started taking high dose slow release melatonin at night, so wrecked the control experiment. I definitely think rapamycin at high doses hampers sleep, which needs a hack, as sleep is HUGE to long term health
Will be doing a baseline of new labs, new gut updated kit microbiome, and then restarting.
MAC INTRAMUSCULAR & INTRANSAL RAPAMYCIN LABS.pdf (55.8 KB)
What’s most notable in the summary is how my TG/FG/hbA1c are relative proxies for trough sirolimus level. The glucose markers change not that significant/interesting in this 6 month update. I’ve had no weight change, no overt signs I’m turning diabetic at 6 months, although a small bump in a still extremely low fasting insulin. I’m strict keto/OMAD, so my AUC glucose/insulin is normally extremely low; starting at the extreme opposite of diabetic/insulin resistant phenotype. Low inflammation, low iron, low insulin, superior physical health…certainly a resistant phenotype. What would happen to me several years on this protocol?
Far more interesting are the lipids and understanding ASCVD risk…something of still raging debate. Does perturbation of lipids by rapamycin raise ASCVD risk? But mTOR inhibition (longevity) comes only with high dose therapeutic, so the all- cause-mortality (ergo lifespan) paradox/dichotomy!
The iron and immuno markers are still at very low normal. Iron saturation is still less 20%, although Ferritin did come up a bit (I stopped donating blood when I started this protocol back in July 2022). The very low iron levels pre therapeutic dose rapamycin put me at much higher “clinical anemia risk” on this protocol, but my long term low iron dumping (and high exercise, oxygen efficiency) seems to have produced a physiological adaptation even to 6 months of high dose rapamycin. Overall, still very much in clinical anemia state, but perhaps reached bottom and uptick. But with washout, will never know trajectory until next and longer term therapeutic intervention. My entire iron journey is suggestive that modern humans are at MUCH HIGHER iron levels than physiologically necessary. For most of evolution and in the 3rd world, people were and are infected with hookworms and parasites that removed iron! Well that’s no longer the case, and modern humans keep building and building iron stores as we age.
“Radioisotope studies with chromium 51-tagged red blood cells have shown that patients with heavy hookworm infection can lose up to 250 ml, or a quarter of a liter of blood, daily, and up to 29 mg of iron in the gastrointestinal tract, thus leading to direct iron-deficiency anemia.”
As iron dumping is one of my central longevity hacks, my n=1 certainly begs the question as to whether modern guidelines should be revisited, separate from the many longevity benefits of low iron.
Diving right in, how is that my TC/LDL/apoaB are relatively UNCHANGED FROM PRE RAPAMCYIN PROTOCOL?? Is this n=1 OR something unique about method of delivery, namely bypassing of 1st pass metabolism by way of IM + IN?
A seminal paper on the impact of therapeutic ORAL dosing rapamycin (10mg/day in general, although individually tailored, trough Sirolimus > 5ng/mL) on renal transplant patients is quite interesting and serves as relative protocol comparison of oral dosing. There are NO healthy persons cohorts taking therapeutic dose rapamycin…I am n=1.
Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients
https://www.jlr.org/action/showPdf?pii=S0022-2275(20)30049-3
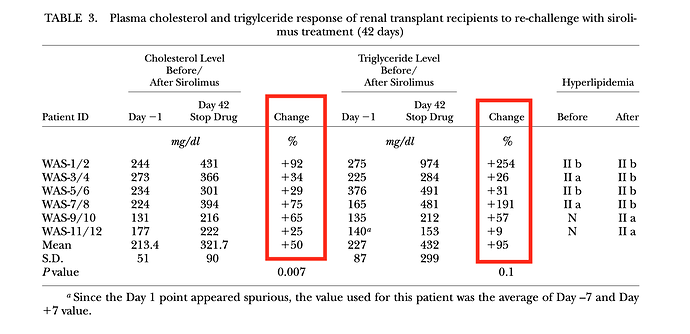
They did a first 14 day dosing discovery trial, and then a second phase of the study, they gave patients daily dosing for 42 days, then measured various lipid markers.
In the 42 day trial, average change in TC and TG was increased 50% and 95%, respectively, although quite a bit of inter-person variability. “In general, triglyceride level were highly responsive to sirolimus dosage. Throughout the entire 6-week treatment period (42 days), sirolimus had no effect on HDL-C levels”
Compare and contrast with my lipids…TC was essentially unchanged, whereas TG did rise by approximately 100%, and HDL remained essentially unchanged vs pre rapamycin dosing baseline.
What about LDL-C? Although the paper didn’t discuss, I extracted the data in a summary table.
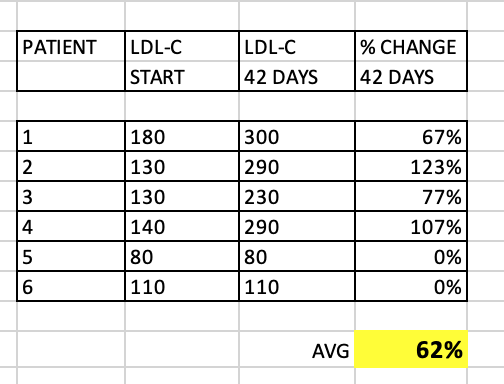
As you can see, quite a significant overall increase in LDL-C (62% average), although some patients had very little change at 42 days. These patients with little change in LDL-C still had significant change in TC/TG. Why would my TC, and for that matter, my LDL-C, remain relatively UNCHANGED?
What about apoB? The study measured apoB-100, which is the same as apoB.
As we know, apoB and LDL-P have a much stronger association to ASCVD, than LDL-C. I extracted the data from the study figures, and prepared a summary table:
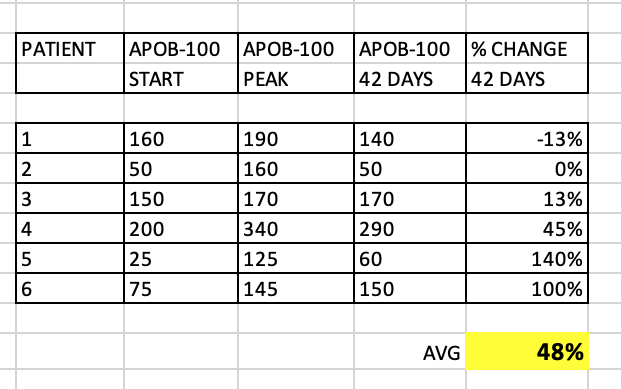
Several patients had a peak in apoB, but the 42 day apoB wildly varied between patients. Some patients had only a small increase, and some over 100%. What’s very interesting is that even the patients with no overall change in LDL-C at 42 days…still had > 100% increase in apoB! Quite the discordance between these 2 lipid markers in this small cohort.
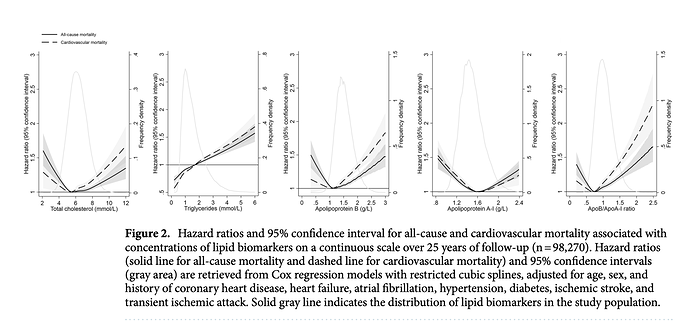
I have 3 apoB measurements during my 6 months, and my level remained essentially unchanged, as did my apoB/apoA1 ratio, itself a marker used in ASCVD. Although a very recent (2021) study showed a U shaped curve to apoB in all cause mortality.
https://www.nature.com/articles/s41598-021-03959-5
Quite interestingly, my apoB and apoB/apoA1 are right at lowest HR. And even with therapeutic high dose rapamycin, my TG of 120mg/dL (1.35 mmol/L) puts me at neutral HR, although the lower the better.
And very interestingly, my LDL-C remained relatively unchanged over the course of the high dose intervention. Why are my TC, LDL-C and apoB resilient to this therapeutic rapamycin dosing…n=1 genetics or method of delivery? Seems less likely n=1 uniqueness given above cohort data that, even though there is clearly VERY potent genetic inter-person variability to rapamycin dosing. Is my liver/genetics that lipids unique to these 6 renal transplant patients? What are the chances I would have a complete outlier of all 3 of TC/LDL-C/apoB unchanged on therapeutic dosing? The protocol? The greater significance long term?
The study also measured apoC-II and apoC-III.
“ApoC-II and apoC-III are important protein components of VLDL and HDL. The plasma levels of apoC-II were typically low and did not change appreciably during the course of the study. In contrast, the initial values of apoC-III were substantial, and increased significantly between day 1 and day 42. Since apoC-II is an activator and apoC-III is an inhibitor of lipoprotein lipase (LPL), these results provide a reasonable explanation for the substantially lower LPL activity in these renal
transplant patients (20–70%) compared with normolipidemic controls.”
“Triglyceride hydrolysis by lipoprotein lipase (LPL), regulated by apolipoproteins C-II (apoC-II) and C-III (apoC-III), is essential for maintaining normal lipid homeostasis. During triglyceride lipolysis, the apoCs are known to be transferred from very low-density lipoprotein (VLDL) to high-density lipoprotein (HDL)”
The study also measured free fatty acids (FFA) as an attempt to further under TG dysregulation and clinical implications. FFA are derived from triacylglycerol by cleavage lipase (in the body).
“These results were supported by measurements of total free fatty acid levels, which indicated considerable expansion of this pool. Taken together, these results support the view that sirolimus enhances the action of hormone sensitive lipase (HSL) and perhaps also inhibits LPL. These effects are the opposite of those mediated by insulin, suggesting that sirolimus may induce hypertriglyceridemia via an insulin-dependent signaling pathway. If the drug interferes with insulin-stimulated triglyceride storage in adipocytes, this could lead to increased release of FFAs into the circulation, their increased uptake by the liver, and increased hepatic secretion of VLDL triglycerides. Alternatively, sirolimus may also decrease FFA oxidation leading to increased FFA availability.”
We know from other animal studies, therapeutic dosing rapamycin massively alters TG metabolism.
https://sci-hub.se/10.1016/j.metabol.2006.01.017
“Rapamycin treatment resulted in more than a 2-fold increase in plasma triglycerides (TG), whereas no differences were observed in plasma cholesterol between RAPA and control groups. Very low density lipoprotein and low-density lipoprotein particles isolated from guinea pigs treated with RAPA were larger in size and contained more TG molecules than particles from control animals. Interestingly, plasma free fatty acids and fasting plasma glucose were 65% and 72% higher in the high-RAPA group than in control. These results suggest that RAPA interferes with TG metabolism by altering the insulin signaling pathway, inducing increased secretion of very low density lipoprotein. In the current study, high plasma TG levels were associated with larger VLDL*** particles carrying an increased amount of TG when secreted by the liver and, subsequently, a larger LDL particle, which was also shown to have higher TG content as compared to the controls. Furthermore, it was observed that the increase in the mean LDL size was mainly due to decrease in smaller, denser fraction of the LDL. This effect of RAPA can be considered positive because smaller LDL is more susceptible to oxidation and increases the risk of coronary heart disease by 3-fold”***
So similar findings to the human renal transplant study, although a fascinating sub analysis of the particle size…MUCH larger on high dose rapamycin. Translatable to human ASVCD risk stratification on high dose rapamycin? Does this trump other lipid markers dysregulated, eg FFA?
And what about elevated FFA…impact on non rapamycin taking cohorts?
“Most obese individuals have elevated plasma levels of free fatty acids (FFA) which are known to cause peripheral (muscle) insulin resistance. They do this by inhibiting insulin-stimulated glucose uptake and glycogen synthesis. FFAs also cause hepatic insulin resistance. They do this by inhibiting insulin-mediated suppression of glycogenolysis. Individuals who are unable to do this (probably for genetic reasons) eventually develop type 2 diabetes. FFAs have recently been shown to activate the IkappaB/NFkappaB pathway which is involved in many inflammatory processes. Thus, elevated plasma levels of FFAs are not only a major cause of insulin resistance in skeletal muscle and liver but may, in addition, play a role in the pathogenesis of coronary artery disease.”
Some references on FFA:
Free fatty acids, cardiovascular disease, and mortality in the Multi-Ethnic Study of Atherosclerosis
Serum free fatty acids are associated with severe coronary artery calcification, especially in diabetes: a retrospective study
Elevation of Free Fatty Acids Induces Inflammation and Impairs Vascular Reactivity in Healthy Subjects
Plasma Free Fatty Acids and Risk of Heart Failure
https://www.ahajournals.org/doi/10.1161/CIRCHEARTFAILURE.113.000521
Plasma Free Fatty Acids and Risk of Heart Failure
https://www.ahajournals.org/doi/pdf/10.1161/circheartfailure.113.000521
Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma biomarkers of endothelial activation, myeloperoxidase and PAI-1 in healthy subjects
Significance of plasma free fatty acid level for assessing and diagnosing acute myocardial infarction
https://sci-hub.st/10.2217/bmm-2019-0291
“Conclusion: An elevated FFA level is an independent risk factor and independent diagnostic marker for AMI.”
In reviewing these papers, the general consensus is that elevated FFA is an independent risk factor for ASCVD. Does the FFA on high dose rapamycin translate to these studies on non rapamycin cohorts? In non rapamcyin cohorts, it certainly suggests higher ASCVD risk.
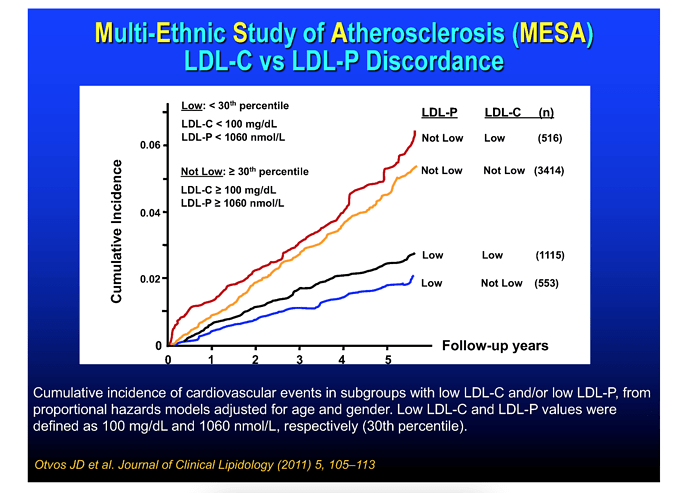
Lastly, let’s take a closer look at apoB and ASCVD risk. More recently the CVD research community has largely moved on from LDL-C as ASCVD prognosticator, and instead looks at small LDL, oxLDL, and LDL-P (LDL particle number, of which apoB is proxy).
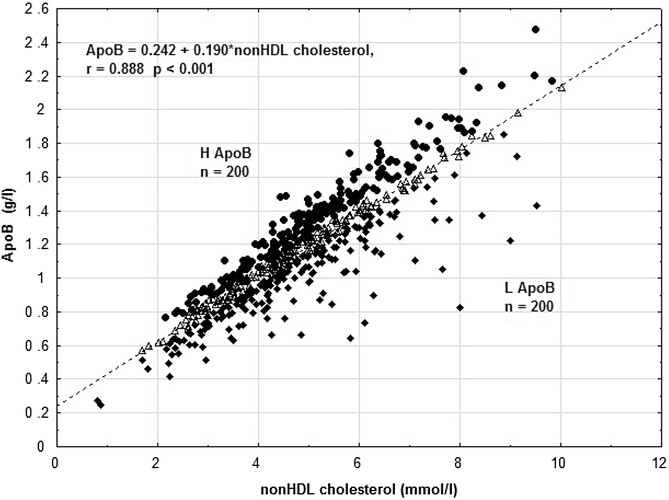
Some reference marker apoB correlation graphs, as most people don’t do a full lipoprotein NMR to get LDL-P…so what can use as good proxies?
https://sci-hub.se/https://doi.org/10.1016/j.jacl.2017.01.020
https://www.dovepress.com/getfile.php?fileID=21661
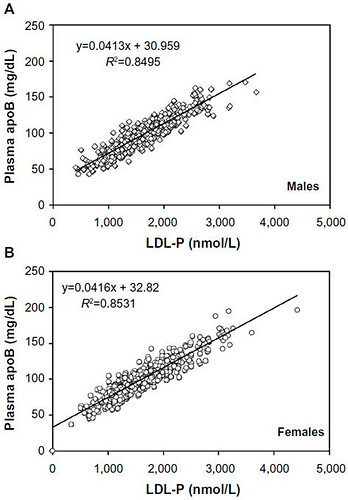
As you can see, apoB is highly correlated to LDL-P.
Attia has many good podcasts on cholesterol and ASCVD that many people follow:
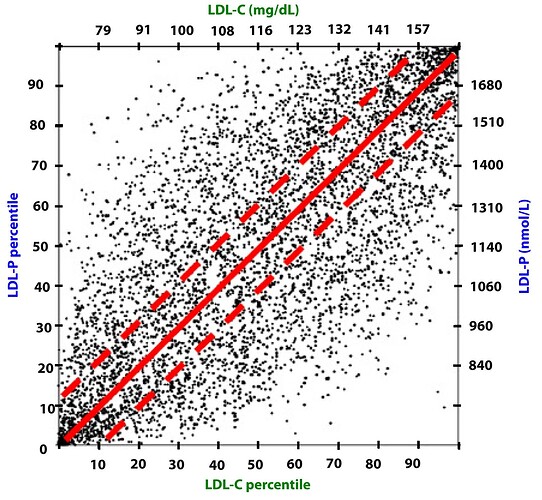
These graphical summaries show clearly a couple of things. Firstly LDL-C and apoB can indeed by DISCORDANT. But LDL-P trumps LDL-C in stratifying risk. We see clearly here that one can have high LDL-C, yet low LDL-P, the net is LOW ASCD risk. In fact, in the 2nd graph, the blue line shows in those with lowest LDL-P, the HIGHER LDL-C is LOWER risk??
Clearly, LDL-C is mostly irrelevant as singular ASVCD risk marker. One must look at the entirety of a person’s phenotype, risk factors, and sub lipoprotein classes TRUMP LDL-C (LDL-P, oxLDL).
In my own n=1 case, I’ve had highish LDL-C even before starting this protocol…looking back from when I started tracking labs 6+ years ago, my LDL-C was around 130, remained similar levels 6 yrs on a PLANT FAT based ketogenic/OMAD diet, and remained stable on 6 months of high dose rapamycin.
I recently (last 2 months) had a cardiologist consult update to baseline ASCVD risk (5 yrs since last update). He rested Lp(a), even though it was very low as from a historical US lab test (one cannot change Lp(a), genetic marker), he wanted his own result (confirmed very low < 10 on upper lab range of 75). He added several ultrasound imaging tests, including femoral, aortic, and peripheral arterial (legs). The results came back CLEAR. Although I’ve had a slight increase my CAC, which is exactly consistent with chronic endurance running (runners paradox), my elevated LDL-C has NOT apparently increased my ASCVD. I also had a full body CT scan as preventative diagnosis, and as an aside, I asked the radiologist to look for whole body arterial calcification. His report “I hope to have this vascular health later in life”. In totality of my clinical makers and phenotype, my cardiologist relayed “whatever you are doing, keep on doing it”. He has not written a statin script, but he’s ITCHING to do it…it’s the standard of care, he needs to cover his butt. I mean, I came to see him and I have elevated LDL, so it’s documented. So clearly, I am n=1 that elevated LDL-C does NOT necessarily correlate with elevated ASVCD and the literature does show there exist higher LDL-C, lower ASCVD risk phenotypes.
Before I restart rapamycin, I will however, be doing FFA , oxLDL, and full NMR lipoprotein labs to baseline these markers in advance of restarting (also as a check of my baseline LDL-P, and how it correlates to apoB. One cannot assume 100% that apoB is LDL-P, sdLDL, or oxLDL proxy; must have the data to check these values (clearly we know genetics/phenotype has huge impact n=1). I can then track these deeper insight markers on therapeutic dosing, and if I do it long enough, re-evaluate ASCVD status with further imaging.
From Attia:
-
LDL-P (or apoB) is the best predictor of adverse cardiac events, which has been documented repeatedly in every major cardiovascular risk study.
-
LDL-C is only a good predictor of adverse cardiac events when it is concordant with LDL-P; otherwise it is a poor predictor of risk.
-
There is no way of determining which individual patient may have discordant LDL-C and LDL-P without measuring both markers.
So my LDL-C and apoB remain UNCHANGED on high dose rapamycin? And what if the raised TG produced larger particle size? Does it mean I didn’t raise my ASCVD risk?
In this washout period, I am experimenting as curiosity, the use of a natural supplement to see how it impacts my LDL-C (perhaps apoB, since I have baseline markers)
Effect of bergamot on lipid profile in humans: A systematic review
“Based on data, 75% of studies showed a significant decrease in total cholesterol, triglycerides and LDLc. The decrease in total cholesterol varied from 12.3% to 31.3%, from 7.6% to 40.8% in LDLc and from 11.5% to 39.5% in triglycerides. Eight trials reported HDLc increase after intervention with bergamot.”
If there is a significant drop in my lipids (will also check apoB), I may continue with this supplement longer term, and then baseline my labs as “pre rapamycin”. Of course, will discuss with my cardiologist to make a final decision.
In summary, my 6 months of therapeutic dose rapamycin has produced a lipids phenotype which is apparently clinically different than therapeutic ORAL dosing rapamycin. Is this superior and clinically significant as rapamycin longevity intervention? Elevated TG definitely remains CENTRAL to both modes of delivery…again, clinically significant? I need more data, longer duration to tease out. UNCHARTED WATERS
The renal transplant/therapeutic rapamycin/lipids study definitely confirms very clearly: everyone will have a UNIQUE response to rapamaycin dosing. And as I’ve stated several times in the past, if you’re not measuring your FULL lab markers such as trough Sirolimus (the morning just before your NEXT DOSE that same morning), lipids, glucose, A1c, full CBC, iron panel, immuno markers, etc, you have NO idea the impact of your dosing protocol. (In fact, for the renal transplant study, they actually had to tweak daily dosing to maintain therapeutic levels, whilst minimizing massive lipid dysregulation in some patients: genetics). Of course, things get amplified in therapeutic dosing protocols re lipid dysregulation, but there are very strong n=1 GENETICS at play.
For those taking CYPA34 inhibitors to boost rapamycin, I understand the rationale to cheaply boost AUC, but as controlled experiment, I doubt very much you can 100% reproduce the AUC spike at every dose/GFJ bolus (variation between fruit, dose volume, speed of gastric remodelling based on say prior meal contents, impact of supplements taking, time of day, etc etc). Certainly, IF you are regularly testing trough Sirolimus, then all is good re getting reproducibility of dosing data insight. As you can see from my data, I also cannot get exact repeated trough Sirolimus at supposed “equal dosing”, although my goal is simply to get above therapeutic dosing (> 5 ng/mL); always achieved.
This is next level sh%t
Thanks, MAC. Your results are exciting and overwhelming at the same time.
@MAC Great summary, thanks for posting all the detail.
Dosing
Your current dosing and trough levels of about 7.5 to 11.1 ng/ML is right in the higher end of the range that organ transplant patients are targeting: " The usual maintenance dose of Sirolimus in these patients is 2 to 5 mg/d and its optimal maintenance trough level is 5 to 10 ng/mL"
So - just be careful with the lower immunity issues. I suspect you are hitting mTORC2 pretty hard with your dosing level and schedule. It is interesting, I’ve seen researchers say that rapamycin really isn’t a very good (strong) immune supressant, and your results seem to confirm that. Dudley Lamming has suggested (generally) that we monitor Tregs levels for a more accurate level of immune system function, is there any chance you can get that tested where you live?
TREGs (T-cell Regulatory Test): (Test details ), Labcorp TREGs test
Sleep
On the Sleep issue, does the Garmin device you use also record REM sleep as distinct from the general sleep score? The reason I ask is that I wonder if rapamycin just changes the the types of sleep you are getting, or the different phases and timing. My experience, and I think most people here, has been that my sleep (admitedly at the lower doses we are using) has always been much better while on rapamycin; deeper, more restful sleep. I’m wondering if its possible that your sleep is more efficient at higher dosing? How are you feeling when you wake up, and during the day? Do you feel well-rested? How is your energy level througout the day?
Do you notice any different during the week after your initial dose? Is day 1 sleep after dosing worse than day 6 sleep? Do you see any difference in the data as blood/sirolimus levels go down over the week?
Heartrate (RHR) and HRV?
Are you tracking RHR and HRV? Have you seen any trends in those during the testing period?
Bloodwork
This will take me a little more time to dig into - but generally looks pretty good. The APOB results are positive.
yea there has been a blockbuster drug that can only be by injection and that is human growth hormone - think call somatropin or something like that.
I can add this panel to my next intervention labs.
Yes, the watch gives STRESS, DEEP, LIGHT, REM, AWAKE metrics. The software, however, does not generate automatic trending views on these metrics, I need to create them manually. I haven’t gone in and exported the raw data to get continuous streams of these, Will circle back.
I went in an extracted a rough cut summary of major trends, and snapshot of 2 specific days: Oct 6, 22 the night of a dose a couple of months into intervention, and Feb 28, 23, during washout.
MAC SLEEP METRICS - 6 MONTHS IM +IN RAPAMYCIN SUMMARY.pdf (463.7 KB)
Anecdotally through all of this, my sense of trending of the data was more stress, less deep, more light, less rem, more awake during intervention. And the general opposite during washout. This is shown in the two snapshot days in the attached. I had no real problem falling asleep…it’s the dynamic during the night. Although I do take high dose magnesium, and my zinc at bedtime…a hack I previously implemented prior to intervention to help with sleep onset.
I never felt tired, prior, during intervention or washout, although I definitely have notes of more yawning in the morning during intervention; although it had NO impact throughout the day or tiredness at night. Some folks have mentioned having a “wired signal” at night…I definitely have notes on this during intervention. In the clinical study literature, insomnia is a side effect of high dose rapamycin.
We do have a thread on it:
In fact, impairing sleep through depressing mTORC1 in the brain has sleep and neurological implications.
Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis
https://sci-hub.se/10.1126/scisignal.aad4949
“Our work supports the hypothesis that one of the functions of sleep is to facilitate protein synthesis We demonstrated that sleep deprivation impaired protein synthesis in the hippocampus using a nonradioactive in vivo translation assay, which was predicted by our previous findings that 5 hours of sleep deprivation lowers the abundance of mTOR in the hippocampus (17). This is consistent with previous findings that rapamycin administration inhibiting mTORC1 impairs sleep-dependent cortical plasticity.”
There is a lot of noise in the day to day sleep data: what I ate, if I drank, whether travelling late night or early AM flights, when I went to sleep. These all had LARGE impacts on my sleep score. Not sure I can definitely answer sleep trending during the week of dosing. To gain deeper insights, I would need more longer term data/analytics, perhaps a more sophisticated sleep tracker, and removing extraneous interventions. I normally keep a very strict sleep hygiene protocol…go to bed same time, complete darkness, wear ear/eye muffs.
As you can see, apparently higher stress levels (less HRV) during intervention, and generally less deep, less REM, more light, more awake: and opposite during washout.
There is a setting in the watch that allows me to get HRV at chosen time durations. I will endeavour to track during next dosing intervention.
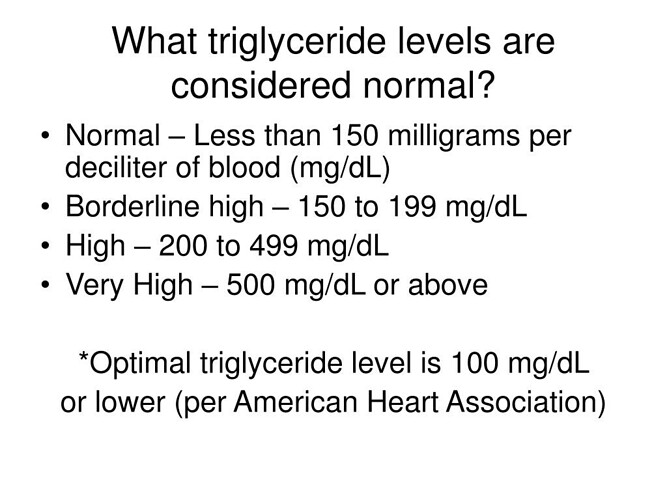
The lack of change in LDL-C and apoB was quite surprising vs pretty much every ORAL rapalog clinical trial, where there is massive lipid dysregulation. The TG elevation is concordant in both oral and IM protocols. I don’t fully understand its ASVCD, metabolic syndrome, or possibly beneficial impacts trending. Re my “elevated TG”, even at 100% more than baseline (128 mg/dL vs 55 mg/dL), this is still below lab upper cutoff of 150 mg/dL: still isn’t anywhere near HIGH or considered hypertriglyceridemia. In fact, my intervention elevated TG would be considered “normal”!!
So no change in TC, LDL-C, apoB, and NORMAL TG…where’s the dyslipidemia from this therapeutic dosing intervention? I’ve never seen this confluence of lipids in any clinical ORAL rapamycin trial. Even in cancer trials, where unlike say transplant cohorts, metabolic dysregulation is not as common, there was massive lipids and glucose dsyregulation on high dosed ORAL rapamycin?
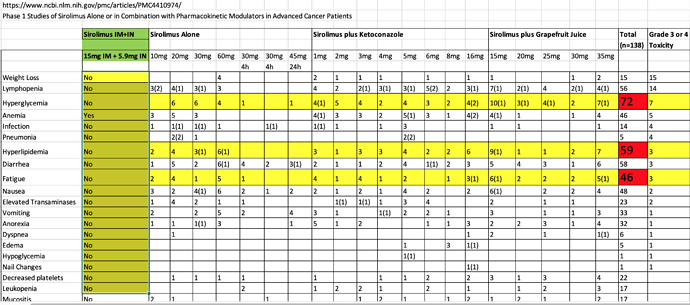
In this seminal cancer/rapamycin trial of high dose oral rapamycin, hyperlipidemia, hyperglycemia, and fatigue were high frequency ADE’s. In fact, there are a very high level of all types of ADE’s (see chart)…I only suffered clinical anemia, and NOT even symptomatic. Surely there must have been many metabolically healthy persons in the study? In the study, nobody could handle high dose without diarrhea. On this forum, I’ve read some members talking about backing off at 20mg/week due to diarrhea. I never suffered diarrhea, just a very soft stool whilst on therapeutic dosing. *How is this possible? Is bypassing 1st pass metabolism with a parenteral method of delivery resulting in a shift of metabolic dysregulation and less ADE’s…THE hypothesis going into this protocol?
Sirolimus-Cancer-GFJ.xlsx (17.9 KB)
In the renal transplant/rapamycin study, the average TG at 42 days therapeutic dosing was 432 mg/dL (one patient reached 974 mg/dL), although these patients were starting from a higher base. Several were above 150mg/dL pre intervention.
Normally, in the obesity and T2D phenotype, elevated TG is a serious dysfunctional state leading to metabolic hell.
What are the complications of high triglycerides?
High levels of triglycerides increase your risk of pancreatitis. This severe and painful inflammation of the pancreas can be life-threatening.
High triglyceride levels also increase your risk of heart and vascular disease, including:
- Carotid artery disease.
- Coronary artery disease and heart attack.
- Metabolic syndrome (a combination of high blood pressure, diabetes and obesity).
- Peripheral artery disease (PAD).
- Strokes.
But in a super healthy person with levels BELOW lab upper cutoff? This TG/FFA rapamycin induced liver/LPL pathway perturbation, how does it mediate the healthspan and longevity signal/outcome?
I am going to have to dig deeper into my FFA and lipoprotein profile with a full NMR to get better insight into LDL-P, sdLDL, and oxLDL. Is this intervention producing a classic pro atherogenic signal or not? I need to find out.
Historically, the rapamycin trial clinicians had pretty much all dyslipidemia patients on lipid management meds (and glucose as well), as standard of care, including in the aforementioned renal transplant study. Consider I took therapeutic dosing for 6 months, yet, my markers did not scream displidemia, glucose dysregulation, nor was I taking ANY meds (statins, metformin, etc)?
Method of delivery? n=1 phenotype?
Hypothetically…what IF I stacked on a statin or PCSK9, and glucose controlling meds to my intervention and lowered all my markers, including, say hypothetically an elevated LDL-P when I re-intervene with protocol?? Crazy thing is my FMD would script all of these things for me, whereas my cardiologist would never script PCSK9 (not indicated), and my regular doc would never script me a SGLT2 inhibitor or Injectable semaglutide (not diabetic).
Stellar labs and crushed mTORC1? Thoughts of longevity escapism?
Back to reality…I will have to do the heavy lifting to try and tease all this out. I need to add even more, deeper insight markers, and longer duration.
I took a look at your labs, what markers led you to think you are near anemic?
I posted on this in another thread.
Moving goal posts for anemia, and iron deficient anemia clinical definitions. In general looking at the totality of my intervention, low iron, low ferritin, low saturation, low hemoglobin, low MCV, high RDW. These are all at/near anemic levels.
My Aug 3, labs, I was clinically iron deficient anemia.
Unless you have been donating blood regularly and/or have very low iron markers, in ill health, and plan to take high dose rapamycin, I wouldn’t worry too much about iron. But of course, you have to monitor everything on rapamycin. If I had continued donating blood on my normal historical 8 week cycle during this high dose intervention, my iron would have tanked much lower (I was near clinical anemia BEFORE starting the intervention), and would have been very interesting to observe if I remained asymptomatic. Just supports my thesis…modern meat eating humans have WAY more iron stored in tissue than blood markers elucidate (excess iron is pro aging). The reference values are far too high. 99% of the population has FAR higher iron stores than me, yet I’m asymptomatic. But I have been adapting to dumping iron for over 5 years and a high endurance exerciser re O2 efficiency…so I am likely not a normal iron physiological reference.
Ferritin is a relatively poor marker. I’d focus on iron plasma, hemoglobin, saturation and your CBC. Don’t forget to test sirolimus trough at every panel.
What are your thoughts about the potential for mTOR2 inhibition?
Amazing overview… because of you MAC I am doing a lot more testing and liking the information it provides… flying a little less blind. DEXA, Coronary Calcium Scan, Labcorp sirolimus trough and post dose levels with GFJ… and without , ApoB… etc.
I highly recommend everyone take your lead and do the testing - look under the hood.
Thanks buddy!!
Somatropin is just one of the cheaper variants of HGH. However, HGH itself isn’t anything new. It’s been used in various therapies for decades, particularly for teens that have low growth hormone (the most famous example being Lionel Messi).
It has a lot of pretty nasty side effects, though, most notably its diabetogenic effect. It’s also something that seems to accelerate aging when tested in various animal models. This doesn’t matter as much to bodybuilders, but to people who want longer lifespan in addition to healthspan, it’s not ideal.
that person who went to the hospital likely did not need to. eventually the infection would have subsided as mine did after using tap water in philippines which i thought was ok but now know it likely was not since i got bad diarhhea from just drinking it for the first time in another place. i always used sterile water at home but did not want to have to take extra weight of baggage so now just use boiled i assume drinking water in PI which most all locals only drink also.
yea i was doing hgh but due to the high cost years ago switched to mk677 which can be taken orally as i do about 20mg/day and much cheaper than hgh.
WHAT THE FUCK! You injected using goddamned tap water? In the Phillippines!?!? How are you even alive? Do you even have the limb you injected into?
And you even got an infection? Did you even go to the damn hospital? What do you mean by “subsided?” Did you literally just wait it out?
For the love of all that is holy, please STOP. You are fucking scaring me. Get help, now.
I’m about to faint.
How many drugs are you pumping into yourself? I’ve followed your posts across several threads, and each additional post you make is somehow more terrifying than the last.
I am normally for harm reduction, but at this point I don’t even think you are mentally sound enough to even listen to what I have to say.
I am not exaggerating when I say this. In all my time in pharma, research, and clinical laboratories, I have never seen a patient as reckless, self-endangering, and absolutely insane as you.
Please, please, please seek medical counsel. You are killing yourself. You’re going to die if you continue this behavior. Get off this forum and go to a doctor!