And a more in-depth analysis of the full paper:
Summary
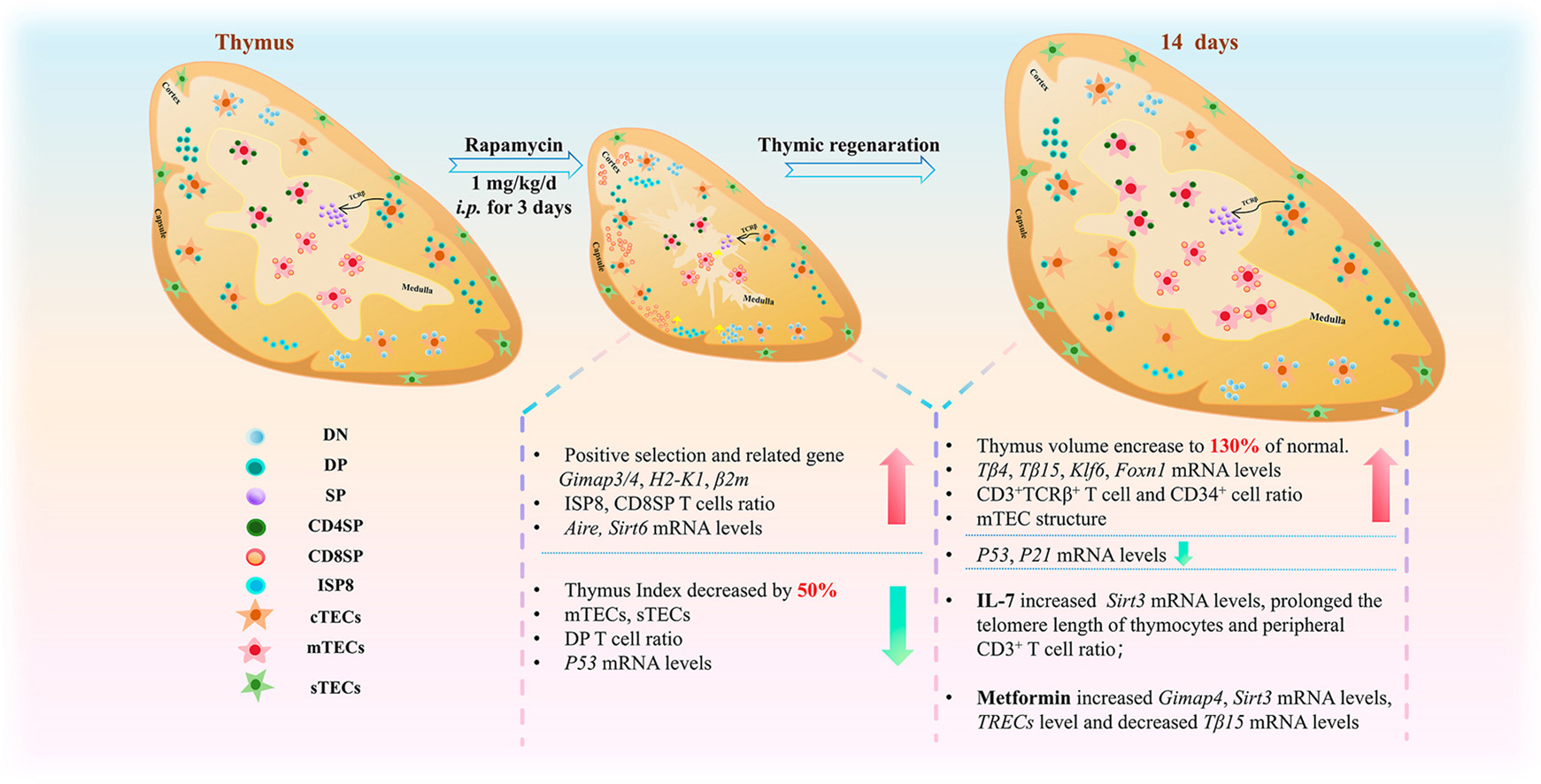
This 2025 study, “Kinetics of thymic regeneration in female mice following short-term rapamycin administration,” by Zhang et al. (Zhejiang Chinese Medical University) investigates how brief exposure to rapamycin (RAPA) influences thymic structure, function, and subsequent regeneration. Female BALB/c mice received 1 mg/kg/day RAPA intraperitoneally for 3 days, and the thymus was analyzed during the involution phase (Day 3) and regeneration phases (Days 3 and 14 after withdrawal). The study also examined whether IL-7 or metformin (MET) could augment thymic recovery.
Key findings:
- RAPA caused acute thymic involution, with ~46% weight loss and cortical/medullary disruption, loss of medullary (mTECs) and subcapsular (sTECs) epithelial cells, reduced double-positive (DP CD4⁺CD8⁺) thymocytes, and shortened thymocyte telomeres.
- During recovery, the thymus over-regenerated to ~130% of baseline mass within 14 days, showing medullary expansion, DP and CD8⁺ SP T-cell rebound, normalized Foxn1/Klf6 expression, and extended thymocyte telomeres.
-
IL-7 modestly promoted telomere elongation and peripheral T-cell recovery, but did not accelerate volumetric thymic regeneration.
-
MET enhanced positive selection (increased Gimap4, Sirt3 expression) and mitochondrial homeostasis, but similarly failed to boost regeneration speed.
- The authors conclude that RAPA triggers transient thymic suppression followed by intrinsic regeneration, potentially explaining its systemic immune-rejuvenating and anti-aging effects.
Novelty
-
Temporal kinetics of thymic remodeling: Previous studies established rapamycin’s general immunomodulatory or rejuvenating effects, but this is the first systematic mapping of thymic involution–regeneration dynamics following short-term RAPA exposure. The demonstration that the thymus rebounds beyond baseline mass (~130%) within two weeks is a novel quantitative finding.
-
Linking telomere dynamics to thymic recovery: The observation that RAPA initially shortens thymocyte telomeres but that regeneration later extends them—especially under IL-7 co-treatment—is a new mechanistic layer connecting mTOR inhibition to cellular aging and DNA maintenance.
-
IL-7 and metformin as modulators of post-RAPA recovery: The combined exploration of IL-7 and MET interventions, showing distinct mechanistic roles (IL-7 → telomere and T-cell homeostasis; MET → mitochondrial function and selection gene expression), adds translational relevance for thymic rejuvenation strategies.
-
Identification of transcriptional markers of regeneration: The integration of Foxn1, Klf6, Gimap4, and Sirt3/Sirt6 expression data provides a molecular timeline for epithelial-thymocyte crosstalk during regeneration.
Critique
Strengths:
-
Comprehensive multimodal design: Combines histology, immunofluorescence, flow cytometry, TEM, and gene expression profiling.
-
Mechanistic integration: The paper links mTOR signaling, TEC biology, telomere integrity, and peripheral immune profiles coherently.
-
Clear temporal resolution: The Day 3 vs Day 14 framework effectively demonstrates biphasic thymic behavior (acute suppression → rebound).
Limitations:
-
Short observation window: Only 14 days of recovery were tracked. It remains unknown whether the over-regeneration is sustained, or followed by secondary involution—important for assessing long-term anti-aging relevance.
-
Single sex and age group: All subjects were 6–8-week female mice; results may not generalize to males or aged cohorts where thymic atrophy is more severe and regenerative potential lower.
-
Functional immunity not directly tested: The study infers improved immune competence from thymic and peripheral markers, but did not measure antigen-specific T-cell responses, infection resistance, or vaccination outcomes.
-
Pharmacologic realism: The RAPA dose (1 mg/kg × 3 days i.p.) produces higher systemic exposure than typical human low-dose regimens (e.g., 3–6 mg weekly orally). Translational extrapolation to clinical dosing remains uncertain.
-
Mechanistic ambiguity: While mRNA data suggest up-/down-regulation of Foxn1, Klf6, Sirt3/6, etc., causality is not demonstrated; loss-of-function or knockdown models would strengthen conclusions about these pathways.
-
Lack of systemic mTOR activity readouts: The study never confirmed mTORC1/2 inhibition in thymic tissue (e.g., via p-S6 or p-Akt assays), which would clarify whether effects were directly mTOR-mediated or secondary to metabolic stress.
Overall Evaluation
This paper provides the first kinetic framework for thymic degeneration and rebound following short-term rapamycin, demonstrating that temporary suppression can paradoxically lead to durable structural and molecular rejuvenation. Its translational implication—that transient mTOR inhibition might “reset” thymic architecture—is conceptually valuable for immune rejuvenation and anti-aging therapy design.
However, the lack of long-term follow-up, male/aged cohorts, and functional immune testing limit its clinical extrapolation. Future work should integrate lineage-tracing, phospho-mTOR readouts, and functional assays of T-cell repertoire diversity and pathogen response.
The paper does not explicitly state the total number of mice used, but it provides sample sizes (“n”) for each experimental comparison, from which the approximate total can be inferred.
From the Methods (Section 2.1) , there were 13 experimental groups :
Nor, Rap, RR1, RR2, RI1, I1, RI2, I2, RML1, RML2, RMH1, RMH2, ML1, ML2 .
Each group corresponds to one treatment/timepoint combination (RAPA ± IL-7 ± MET at 3 days or 14 days).
Across the Results sections, the figures consistently report:
-
n = 6 mice for most histological, mRNA, and telomere assays (Figs 1 – 6)
-
n = 3 for flow-cytometry subsets (T-cell phenotyping) and some peripheral-blood assays.
Given that multiple endpoints were measured from the same animals per group (not separate cohorts), the main analysis likely used ~6 mice per group.
 Approximate total:
Approximate total:
13 groups × 6 mice ≈ 78 mice total,
with some sub-assays (flow cytometry) using 3 mice per group.
So, while the paper never provides a single total-animal count, the data indicate that around 70–80 female BALB/c micewere used in total.
Journal quality snapshot
The article appears in European Journal of Pharmacology (Elsevier). Current public metrics (2024/25) place it in a solid, field-standard tier:
- Journal Impact Factor (Web of Science, 2024): ~4.7.
- SCImago SJR (2024): 1.197; Quartile: Q1 in Pharmacology & Pharmacy.