I agree (I didn’t know before btw, learned this thanks to Neo in another thread; I love this community where I learn something new every single day!)
For PD, we have RCT + MR + longitudinal + observational studies showing a decline with statins. But that’s in PD. In healthy individuals, RCTs don’t show anything indeed. But given the link between PD and LBD I found it interesting that this paper looked at LBD. Given the time needed for NDDs to develop and the specific demographic (old people), this cannot be captured by short RCTs on the general population. Do we have RCTs of statins on people with MCI? I’m pretty sure that people with MCI are excluded from most trials because they cannot follow the protocol, and it would be a mess to have them in. I could only find these trials:
- Trial of Simvastatin in Amnestic Mild Cognitive Impairment (MCI) Patients (SIMaMCI): Unknown status…
- Evaluate the Effect of Atorvastatin on Cerebrovascular Reactivity in MCI: Results next year?
- Statins In The Elderly (SITE): results soon? (quite a crazy trial: it’s about stopping statins in people above 75yo and seeing the results 3y later…)
- A Clinical Trial of STAtin Therapy for Reducing Events in the Elderly (STAREE) (STAREE): results in 2026?
- Pragmatic Evaluation of Events And Benefits of Lipid-lowering in Older Adults (PREVENTABLE): results in 2027?
Regarding the STAREE trial, the authors noted in early 2023:
(source)
Yes, 740k is massive. But at the end for LBD it’s 2,591 cases: 4,027 controls. And people with LBD are often undiagnosed or misdiagnosed (source). So there are probably many LBD cases that are not LBD but another kind of dementia or depressive disorder while there might be many LBD cases among controls. How many? I don’t know but that’s why (and combined with the signal for PD mentioned above) I’m not “as strict” in terms of CI and I’m looking for “directions”.
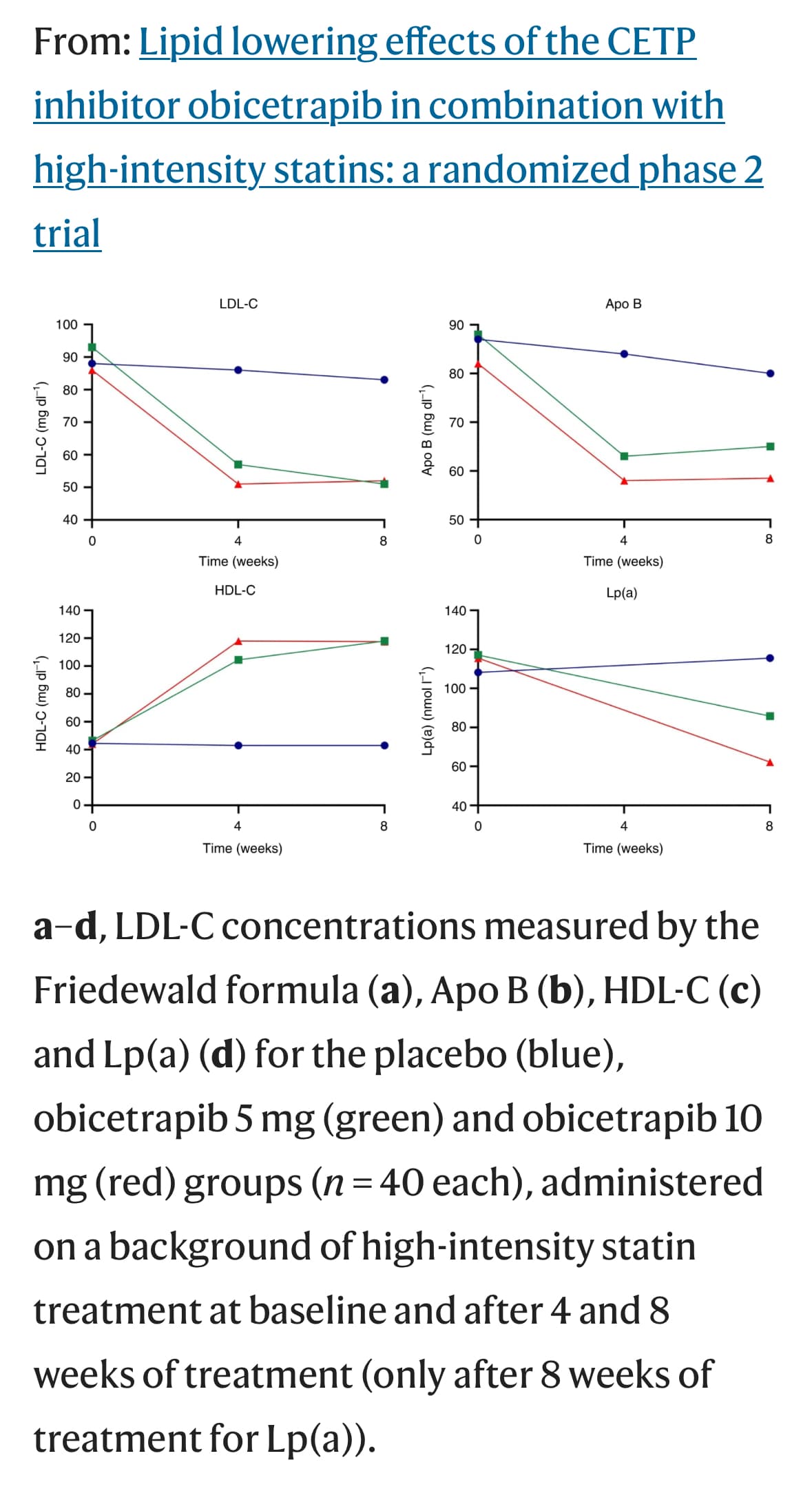
Hm… They write “Conversely, we observed several adverse associations for HMGCR inhibition with lowered cognitive performance (beta: –0.082; 95% CI: –0.16 to –0.0080; P = 0.03), reaction time (beta = 0.00064; 95% CI: 0.00030-0.00098; P = 0.0002), and cortical surface area (beta = –0.18; 95% CI: –0.35 to –0.014; P = 0.03).” and “However, our finding of concurrent improved memory performance associated with the HMGCR instrument highlights the complexity of the HMGCR-cognition relationship.” and for these, we can see on the image that for statins (HMGCR): cognitive performance is on the left of the axis, memory performance is on the right, reaction time is on the right, but they used “reaction time DEFICIT” (why on earth did they do that?!), and total cortical surface area is on the left. So I understood that left = negative beta = “bad”. Did I misunderstand? ![]() Because then they write: “genetic inhibition estimates for PCSK9 were on the beneficial side of null with regression coefficients in the direction of reduced AD progression score, lower amyloid deposition levels, and lower LBD risk” And for PCSK9 they give (Table 2):
Because then they write: “genetic inhibition estimates for PCSK9 were on the beneficial side of null with regression coefficients in the direction of reduced AD progression score, lower amyloid deposition levels, and lower LBD risk” And for PCSK9 they give (Table 2):
- Disease progression score: 0.0086 (–0.16 to 0.17)
- Cerebral amyloid deposition: –0.0038 (–0.55 to 0.55)
- Lewy Body dementia: –0.018 (–0.51 to 0.48)
“on the beneficial side of null” seems quite a big leap of faith but anyway, it means that negative beta for LBD is protective? So statins potentially protective?
Help needed if anyone knows how to understand the results…
Btw, from last week at the Pan American Parkinson’s Disease and Movement Disorders Congress. So it’s not only about me/us a few longevity weirdos here. Some serious folks also have doubts: https://twitter.com/AlbertoEspay/status/1756678599438995951