But You missed the main point - “We did not use dealcoholized red wine in human studies because of the very low palatability. Our nuns refused to take the non-alcoholic red wine.” Who said the penguins don’t have good taste?
Looking at this study again (by US NIH + Oxford researchers in the JACC, so most likely high quality), I’ve just noticed the figure: although it didn’t reach statistical significance it seems that statins may be good for amyloid but bad for Lewy body dementia. (while PCSK9i are neutral for both) Do I understand things correctly? So it might explain the contradictory results in studies around statins and cognition. Could it be that for instance statins reduce the risk of vascular dementia and AD but increase the risk of PD and PDD/DPB/LBD? (pure guess) So they would be overall neutral on “dementia” but with different outcomes by subtype, and because it’s so hard to diagnose the subtypes (and people often have mixed dementia with all pathologies present) it could blur the results in studies. Please correct me if I’m wrong.
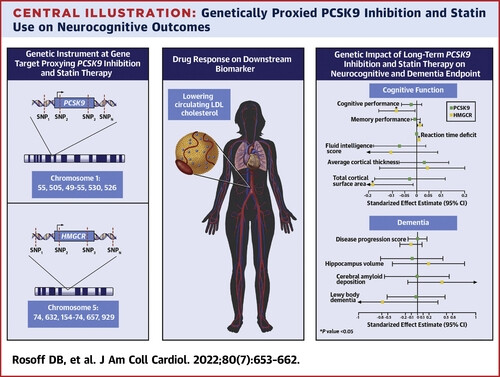
First, an important caveat to MR studies is that they can reflect influences of the genes during development, which don’t apply for drugs that are initiated during midlife. Low HMGCR activity could plausibly restrain myelination or other effects on the rapidly-growing fetal brain in ways that are irrelevant in the very slow turnover state in mid- and late-life.
We use MR best as a proxy for clinical trials we can’t run. We’ve run lots of clinical trials with statins: there’s no ‘there’ there.
Second, in an MR of ∼740,000 participants, the CIs for both amyloid deposition and DLB extend so far on both sides of 0 that you can in any case conclude with substantial confidence that there’s no ‘there’ there on its basis alone.
Wouldn’t the interpretation be the opposite based on the graph?
However, I agree with @RapamycinCurious that speculating is challenging given the lack of statistical significance in the associations.
Personally, if genetically predisposed to any type of dementia, I would opt to avoid statins though.
Investigating MR with cognition is a futile effort. It is a highly polygenic trait so a single genes have very small effects in either direction. The good thing is that almost nothing will affect cognition in the long term in a clinically significant way. Including PCSK9 inhibition and HMCGR, even with lifelong inhibition.
All-cause dementia and mortality is interesting however.
I agree (I didn’t know before btw, learned this thanks to Neo in another thread; I love this community where I learn something new every single day!)
For PD, we have RCT + MR + longitudinal + observational studies showing a decline with statins. But that’s in PD. In healthy individuals, RCTs don’t show anything indeed. But given the link between PD and LBD I found it interesting that this paper looked at LBD. Given the time needed for NDDs to develop and the specific demographic (old people), this cannot be captured by short RCTs on the general population. Do we have RCTs of statins on people with MCI? I’m pretty sure that people with MCI are excluded from most trials because they cannot follow the protocol, and it would be a mess to have them in. I could only find these trials:
- Trial of Simvastatin in Amnestic Mild Cognitive Impairment (MCI) Patients (SIMaMCI): Unknown status…
- Evaluate the Effect of Atorvastatin on Cerebrovascular Reactivity in MCI: Results next year?
- Statins In The Elderly (SITE): results soon? (quite a crazy trial: it’s about stopping statins in people above 75yo and seeing the results 3y later…)
- A Clinical Trial of STAtin Therapy for Reducing Events in the Elderly (STAREE) (STAREE): results in 2026?
- Pragmatic Evaluation of Events And Benefits of Lipid-lowering in Older Adults (PREVENTABLE): results in 2027?
Regarding the STAREE trial, the authors noted in early 2023:
(source)
Yes, 740k is massive. But at the end for LBD it’s 2,591 cases: 4,027 controls. And people with LBD are often undiagnosed or misdiagnosed (source). So there are probably many LBD cases that are not LBD but another kind of dementia or depressive disorder while there might be many LBD cases among controls. How many? I don’t know but that’s why (and combined with the signal for PD mentioned above) I’m not “as strict” in terms of CI and I’m looking for “directions”.
Hm… They write “Conversely, we observed several adverse associations for HMGCR inhibition with lowered cognitive performance (beta: –0.082; 95% CI: –0.16 to –0.0080; P = 0.03), reaction time (beta = 0.00064; 95% CI: 0.00030-0.00098; P = 0.0002), and cortical surface area (beta = –0.18; 95% CI: –0.35 to –0.014; P = 0.03).” and “However, our finding of concurrent improved memory performance associated with the HMGCR instrument highlights the complexity of the HMGCR-cognition relationship.” and for these, we can see on the image that for statins (HMGCR): cognitive performance is on the left of the axis, memory performance is on the right, reaction time is on the right, but they used “reaction time DEFICIT” (why on earth did they do that?!), and total cortical surface area is on the left. So I understood that left = negative beta = “bad”. Did I misunderstand? ![]() Because then they write: “genetic inhibition estimates for PCSK9 were on the beneficial side of null with regression coefficients in the direction of reduced AD progression score, lower amyloid deposition levels, and lower LBD risk” And for PCSK9 they give (Table 2):
Because then they write: “genetic inhibition estimates for PCSK9 were on the beneficial side of null with regression coefficients in the direction of reduced AD progression score, lower amyloid deposition levels, and lower LBD risk” And for PCSK9 they give (Table 2):
- Disease progression score: 0.0086 (–0.16 to 0.17)
- Cerebral amyloid deposition: –0.0038 (–0.55 to 0.55)
- Lewy Body dementia: –0.018 (–0.51 to 0.48)
“on the beneficial side of null” seems quite a big leap of faith but anyway, it means that negative beta for LBD is protective? So statins potentially protective?
Help needed if anyone knows how to understand the results…
Btw, from last week at the Pan American Parkinson’s Disease and Movement Disorders Congress. So it’s not only about me/us a few longevity weirdos here. Some serious folks also have doubts: https://twitter.com/AlbertoEspay/status/1756678599438995951
Yes, both psk9 and hmgcr inhibition genes associated with lower mean lewy body disease risk. But without any statistical significance. So potentially statins still could be protective or harmful or neutral
Thanks. Still interesting because another recent MR (preprint) showed that statins may be neutral wrt PD risk but might shift from the freeze of gait subtype to the tremor subtype. Maybe one of these subtype is more associated with cognitive decline than the other.
Anyway, there’s a link between lipid lowering drugs and cognition and it’s probably not as simple as “statins good” or “statins bad” (does it depend on your BMI? Diabetes status? Genes? Age? ![]() ). I hope the ongoing trials will clarify the picture. I contacted the lead clinician behind the statin cessation study (Statins In The Elderly (SITE)): results expected “this summer”. Wait and see…
). I hope the ongoing trials will clarify the picture. I contacted the lead clinician behind the statin cessation study (Statins In The Elderly (SITE)): results expected “this summer”. Wait and see…
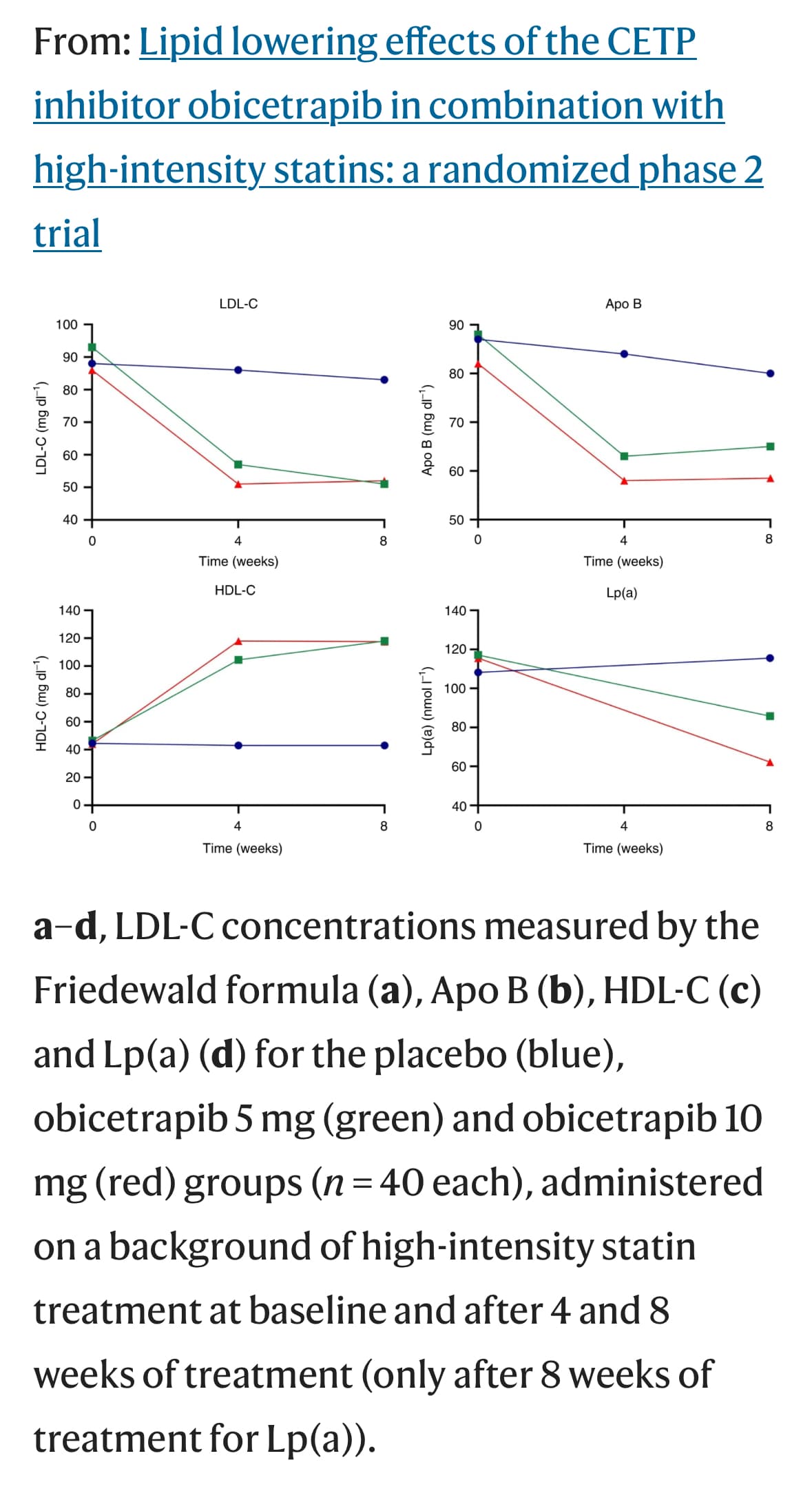
Obicetrapib might come to market the latter part of this year if approved for reducing LDL, based on two phase 3 trials:
“HeFH and ASCVD can be devastating diseases which, if inadequately addressed, can result in myocardial infarction, cerebral infarction, or cardiovascular death,” said John Kastelein, M.D., Ph.D., FESC, Chief Scientific Officer of NewAmsterdam. “It has become increasingly clear that lower levels of LDL-C are directly correlated with a reduced risk for major adverse cardiovascular events. With obicetrapib, we aim to deliver LDL-C reductions that are substantially better than currently available non-statin oral therapies, in a convenient, tolerable formulation. We are pleased to have both BROADWAY and BROOKLYN, the two pivotal Phase 3 trials necessary to support a potential LDL regulatory filing, fully enrolled and look forward to reporting data from both studies in the second half of 2024.”
Our product candidate, obicetrapib, is a next-generation, oral, low-dose CETP inhibitor that we
are developing to potentially overcome the limitations of current LDL-lowering treatments. We believe
that obicetrapib has the potential to be a once-daily oral CETP inhibitor for lowering LDL-C, if
approved. In our Phase 2b ROSE trial, obicetrapib demonstrated a 51% lowering of LDL-C from
baseline at a 10 mg dose level on top of high-intensity statins. In all three of our Phase 2 trials, TULIP,
ROSE and OCEAN, evaluating obicetrapib as a monotherapy or a combination therapy, we observed
statistically significant LDL-lowering activity combined with generally moderate side effects and no
drug-related, treatment-emergent serious adverse events (“AEs”). Obicetrapib has demonstrated strong
tolerability in more than 600 patients with low or elevated lipid levels (“dyslipidemia”) in our clinical
trials to date. Obicetrapib is also expected to be relatively low in cost to manufacture compared to most
other branded LDL-lowering therapies on the market with high efficacy. We believe that the estimated
low cost of goods for obicetrapib will enable favorable pricing and position it to significantly improve
patient access to a high efficacy LDL-lowering therapy compared to existing non-statin treatments.
Furthermore, we believe that obicetrapib’s oral delivery, demonstrated activity in low doses, chemical
properties and tolerability make it well-suited for combination approaches. We are developing a fixed
dose combination of obicetrapib 10 mg and ezetimibe 10 mg, which we believe will demonstrate even
greater potency
We believe that CETP inhibition may also play a role in other indications by potentially mitigating
the risk of developing diseases such as Alzheimer’s disease or diabetes. Evidence suggests that
cholesterol accumulation in the brain is a precursor to Alzheimer’s disease. For example, rodents lack
the CETP gene and are resistant to Alzheimer’s disease. In early preclinical studies, when the human
CETP gene is knocked into a mouse, the cholesterol content of the mouse brain was observed to increase
by 25% and when combined with the gene for the amyloid precursor protein, hypothesized to be a driver
of Alzheimer’s disease, the risk of developing Alzheimer’s disease may greatly increase. In a preclinical
study, we observed that CETP inhibition promoted cholesterol removal from the brain and improved
cognition. We commenced a Phase 2a trial in early 2022 in patients with early Alzheimer’s disease and
the apolipoprotein E4 (“ApoE4”) mutation to evaluate the pharmacodynamic and pharmacokinetic
effects, safety and tolerability of obicetrapib. Clinically demonstrated anti-diabetic benefits have been
observed with CETP inhibition in Phase 3 CVOTs that, if seen in obicetrapib, would differentiate it from
current treatment alternatives, especially statins. We are planning preclinical studies to examine the
potential of obicetrapib for patients suffering from diabetes and have included the onset of diabetes as
an endpoint in our PREVAIL CVOT, as measured by AEs indicating Type 2 diabetes, initiation of anti-
diabetes medication after confirmed diabetes diagnosis or high levels of hemoglobin A1c and fasting
plasma glucose.
https://ir.newamsterdampharma.com/static-files/91cc75c7-08db-4696-9bd6-c05bb01762ed
Super interesting. This preprint MR confirms the potential for dementia prevention: https://www.medrxiv.org/content/10.1101/2023.11.03.23298058v2
Hopefully we can see their 2023 annual report soon?
Also promising Lp(a) it seems (even if I know there are other meds for that also reading out soon that look promising).
Obicetrapib 5 mg reduced ApoB by 24.4%, non-HDL by 38.9% and Lp(a) by 33.8%, whereas obicetrapib 10 mg reduced ApoB by 29.8%, non-HDL by 44.4% and Lp(a) by 56.5% (P for all < .0001), Davidson and colleagues found.
And also beyond Lp(a) and Apo B effects - increase in HDL (that they are not focusing communication on as people are skeptical about past attempts to HDL up…)
I would be surprised if that didn’t publish and pass peer review. It is after all the scientists at NewAmsterdam Pharma like Kastelein.
I don’t understand why they didn’t look at other dementia like for Alzheimer’s, instead specifically PD dementia and LBD. The effect on PD dementia was nice though.
Yes great team so can’t wait to see it published. For AD they write: “Given the observed APOE4 interaction with CETP, and the absence of APOE4 stratified GWAS we did not explore associations with AD.”
Great, I was worried for a second. So far this drug seems very promising. CETP target also have a bunch of old papers from longevity researchers like Barzilai.
What’s the gist of the longevity impact (outside of cardiovascular)?
Dunno I didn’t bother reading them as they are probably using outdated methodologies.
I could take a look at a later time and compile a lot of evidence for the Obicetrapib fanclub.
It has potential to be a longevity blockbuster drug.
Hello Lara, old post but may I suggest Intermittent Fasting. Please view YouTube videos by Pradeep Jamnadas. My blood results improved after 6 months or so of IF. It was well worth it, please persevere.
I agree about IF and usually follow it naturally for as long as I remember myself. I stop eating not later than 7 pm and have my firsts meal around 9-10 am. My lipids improved much after I consistently took rosuvastatin, 5 mg, and ezetimibe.
FWIW
You do not like Calley Means message/comments.
As he tell’s the truth.