Most of the people who have participated in rapamycin clinical trials have been very sick, organ transplant patients who are typically taking many different drugs. So we don’t have much data on these risks in healthy people taking rapamycin.
What data we do have in terms of potential health risks of rapamycin comes from continuous dose rapamycin in mice, and very high continuous doses used in transplant and cancer patients. Neither of these situations translate well to lower dose, pulse-dosing of rapamycin that is typical in anti-aging applications.
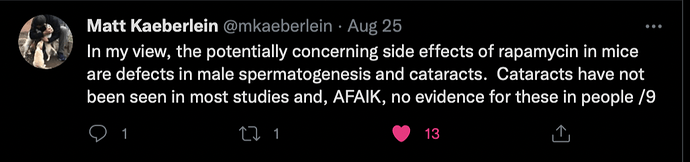
Two adverse outcome risks that have been seen in mice (cataracts) and transplant patients (lower testosterone and spermatogenesis) have been identified by Prof. Matt Kaeberlein as being potentially concerning.
The increased risk of cataracts is seen in mice that are daily dosed at higher levels of rapamycin. Decreased trends in testosterone and spermatogenesis has been seen in mice and transplant patients who have used rapamycin daily, but the effects seem to be similar to that seen in caloric restriction (perhaps an evolutionarily conserved response to low food availability; when food supplies are low, mammals need to focus on survival and not procreation). As with caloric restriction, when daily high doses of rapamycin are stopped, testosterone and spermatogenesis seems to return to normal.
Experiments in rats using sirolimus have shown no effects on fertility in females, and in male rats, there was no significant difference in fertility rate compared with the controls at a dosage of 2 mg/kg (approximately 4–11 times the clinical doses adjusted for body surface area in humans). However, reductions in testicular weight and/or histological lesions (e.g. tubular atrophy and tubular giant cells) were observed in rats following the dosages of 0.65 mg/kg (approximately 1–3 times the [organ transplant]clinical doses adjusted for body surface area in humans) (source)
Regarding sperm count, reduction was observed in male rats following the administration of sirolimus for 13 weeks at a dosage of 6 mg/kg (approximately 12–32 times the clinical doses adjusted for body-surface area), but the counts showed improvement by 3 months after dosing was discontinued (source)
Potential Issues to Watch For: Impaired Gonadal Function / Spermatogenesis and Cataracts
Mice treated with continual dosing rapamycin starting at 9 months of age have significantly higher incidence of testicular degeneration and cataracts; harmful effects of this kind will guide further studies on timing, dosage, and tissue-specific actions of rapamycin relevant to the development of clinically useful inhibitors of TOR action.
Source: Rapamycin slows aging in mice
Neff et al. (2) conducted a comprehensive histopathological analysis of old mice and found that precancerous lesions were significantly reduced by rapamycin, while other age-related pathological lesions (including cataracts, which are often used as a measure of aging) were not significantly altered. Wilkinson et al. (11) reported that rapamycin reduced a number of histopathology endpoints in the heart, liver, adrenal glands, and endometrium in old mice; however, cataracts were increased.
Source: Rapamycin, anti-aging, and avoiding the fate of Tithonus
Rapamycin and Your Gonads:
Sirolimus and everolimus inhibit the activity of mammalian targets of rapamycin, a serine/threonine kinase involved in numerous cell-growth processes. Molecular mechanisms of action of TOR-I on the testis involve inhibition of a stem cell factor/c-kit-dependant process in spermatogonia. Preliminary results appear to show that TOR-I treatment has deleterious actions on the testis and impairs gonadal function after renal transplantation, but the impact of these effects are unknown.
The authors confirmed that total testosterone was lower and FSH and LH higher in the group of patients treated with sirolimus than in those from the control group, and in multivariate analysis, only the use of sirolimus was significantly correlated with decrease of testosterone (age, race, etiology of renal failure, transplant and dialysis durations, antihypertensive and non-mammalian target of rapamycin-I immunosuppressive treatments not significant). However, even though the IIEF score was slightly lower in the sirolimus group, there was no significant difference in sexual score between the two groups (mean IIEF score: 49/75 in the sirolimus group; 52/75 in the control group). Moreover, free testosterone levels did not differ significantly between the two groups.
Regarding the impact of sirolimus on sperm, there was a recently published a case report on a 36-year-old male kidney-transplant recipient treated by an immunosuppressive regimen that contained sirolimus. He presented with dramatic, reversible sperm impairment. He initially (for 3 months) 2 mg/day of sirolimus associated with 10 mg/day of prednisone, 200 mg/day of cyclosporine, and then for 33 months 7 mg/day of sirolimus and 10 mg/day of prednisone. During this time he could not get his wife pregnant. When he stopped taking sirolimus, he could and did get his wife pregnant.
Full Paper: Gonadal impact of target of rapamycin inhibitors
huyghe2007.pdf (483.6 KB)