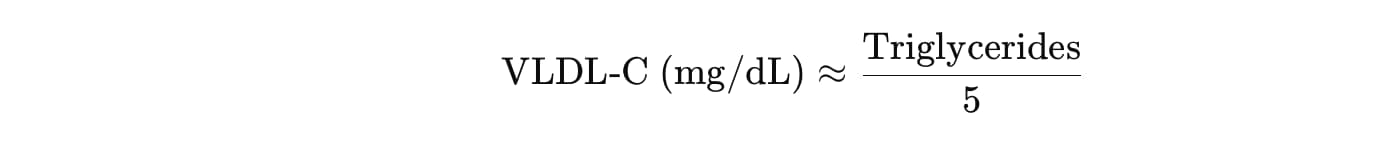Someone needs to be doing this type of in-depth analysis on an ongoing basis on the 500 to 1000 longest lived people in the world.
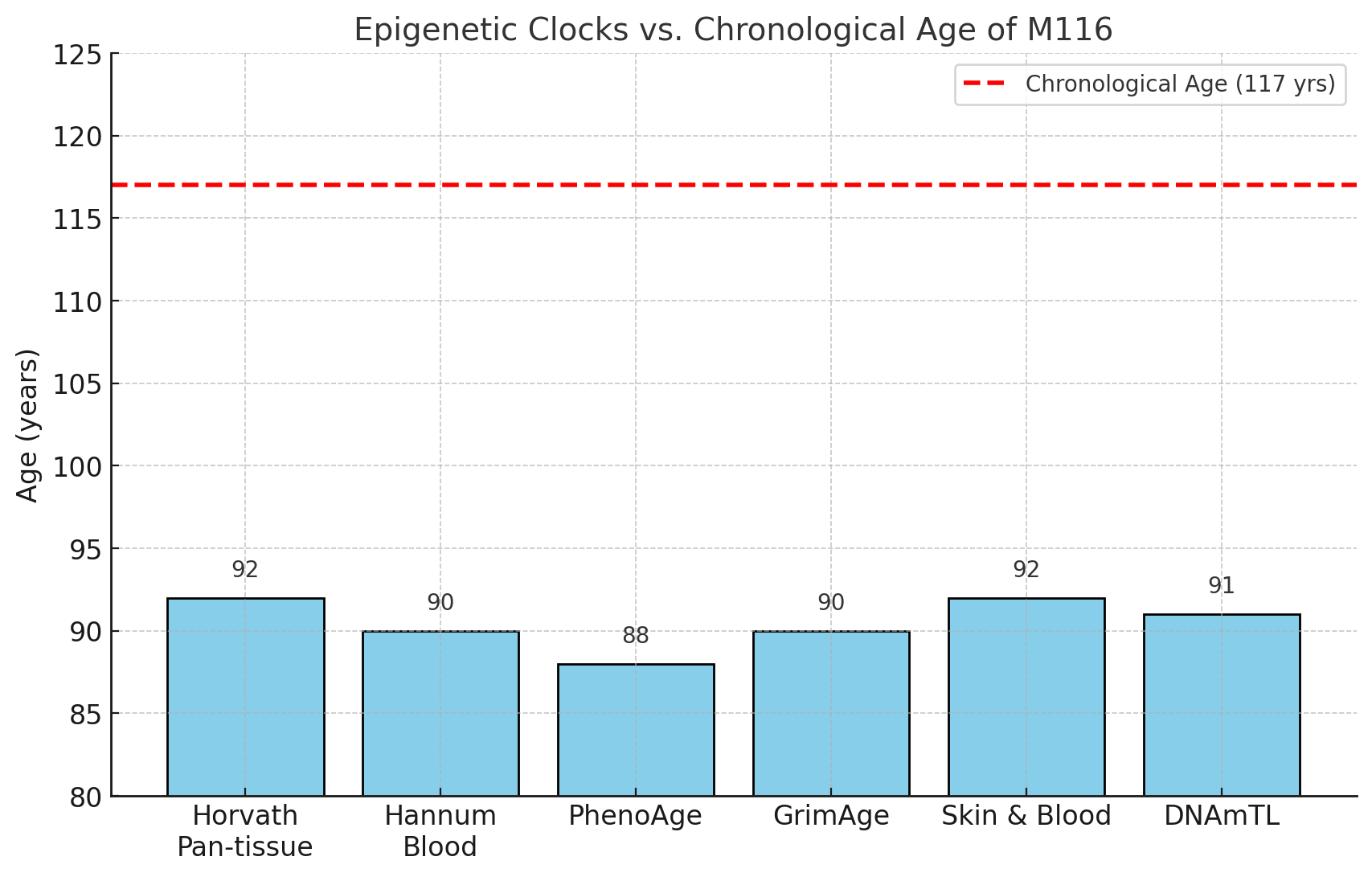
Between 17 January 2023 and 19 August 2024, María Branyas Morera, of Spain, was officially the world’s oldest person, until she died aged 117 years and 168 days. To uncover the secrets of her extraordinary longevity, a team of researchers has done a deep dive into her genetics, microbiome and lifestyle.
When Morera was 116 years old, the team collected samples of her blood, saliva and stool, to analyse her genetics. “She had an exceptional genome enriched in variants in genes that are associated with enhanced lifespan in other species, such as dogs, worms and flies,” says team member Manel Esteller at the Josep Carreras Leukaemia Research Institute in Barcelona, Spain.
Morera, who showed no sign of dementia, also had many gene variants that keep blood lipid levels low, protecting the heart and cognition, says Esteller. “At the same time, she was devoid of gene variants associated with the risk of pathologies such as cancer, Alzheimer’s and metabolic disorders.”
Blood test results also revealed that her lipid metabolism was among the most efficient ever reported, according to the researchers. “Her lipid profile was amazing, with very low cholesterol,” says Esteller. “This was related to her frugal diet and genes that quickly metabolised damaging molecules.”
Esteller says Morera didn’t drink alcohol or smoke, and adhered to a Mediterranean diet, made up of lots of vegetables, fruits, legumes and olive oil. She also ate three servings of plain, sugar-free yoghurt every day.
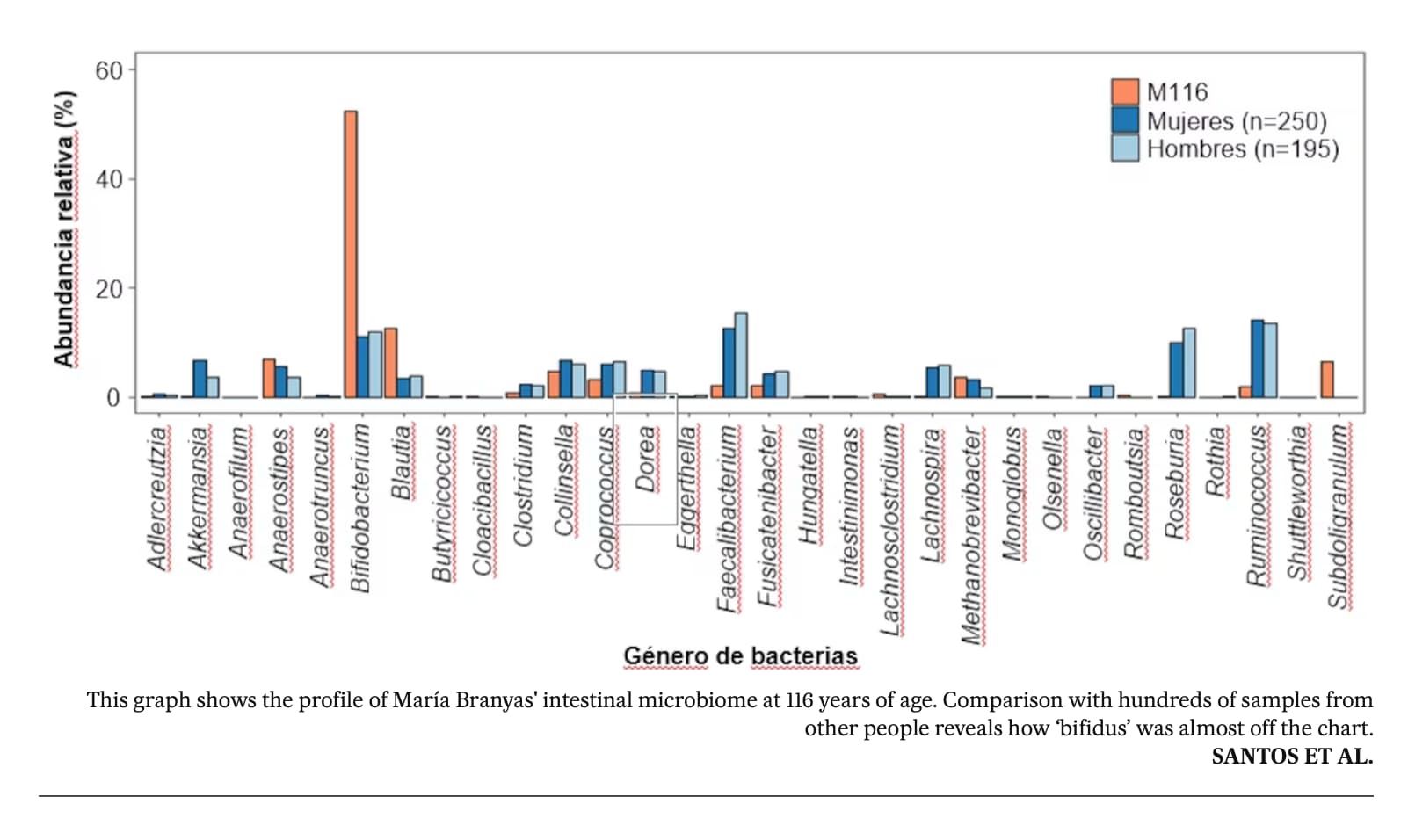
One of the most “startling” findings was the high levels of Actinobacteriota bacteria in her gut, including the well-known probiotic Bifidobacterium. This typically declines with age, but is elevated in centenarians and supercentenarians, and it is thought to have multiple anti-ageing benefits, such as reducing inflammation.
The team suspects her yoghurt consumption helped continuously replenish her Bifidobacterium levels. “It shows that maybe a dietary intervention can be associated not only with avoiding obesity and other pathologies, but also with prolonged life, acting through the microbiome gut landscape,” says Esteller.
Read the full story: World’s oldest person had a young microbiome and ‘exceptional genome’ (NewScientist)
She lived to 117: what her genes and lifestyle tell us about longevity (Nature Aging)
Branyas aced her bloodwork; she had low levels of ‘bad’ cholesterol and high levels of ‘good’ cholesterol, which suggests an efficient lipid metabolism. Her inflammation markers were also low and she had a strong immune system — at the age of 113, she was the oldest person in Spain to have COVID-19 and survive. “Sometimes our immune cells get a little bit unloyal and start to attack our own cells, causing inflammation,” Esteller says. “There was no nothing like that.”
Esteller thinks Branyas’s lack of inflammation could be linked to her healthy gut microbiome, which resembled that of a much younger person. He points to her high levels of Bifidobacterium, a genus of beneficial bacteria that was probably boosted by her diet, which included three daily servings of yogurt.
Richard Faragher at the University of Brighton in the UK says the study demonstrates how many tests are now available to longevity researchers, but cautions that this is a case report of one individual and there is a “danger that a slightly unusual variant in, for example, a gene can be turned into a scientific ‘just-so story’ about its relevance to ageing”.
Full story: She lived to 117: what her genes and lifestyle tell us about longevity (Nature Aging)
Full research paper (Open access): The multiomics blueprint of the individual with the most extreme lifespan (Cell)