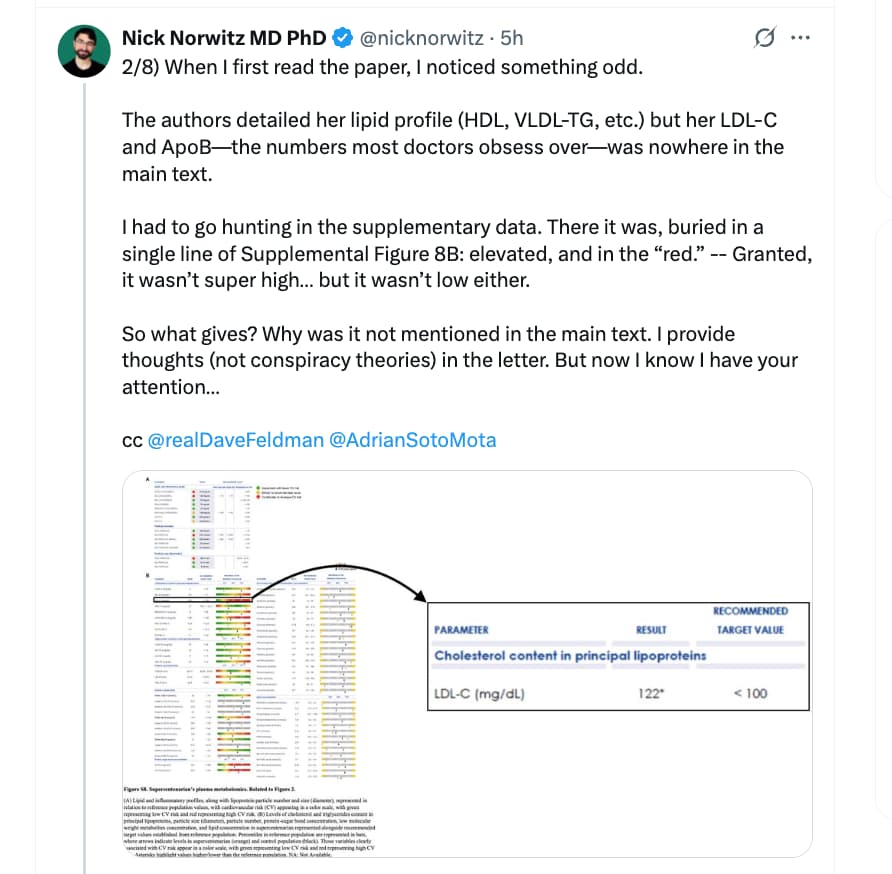
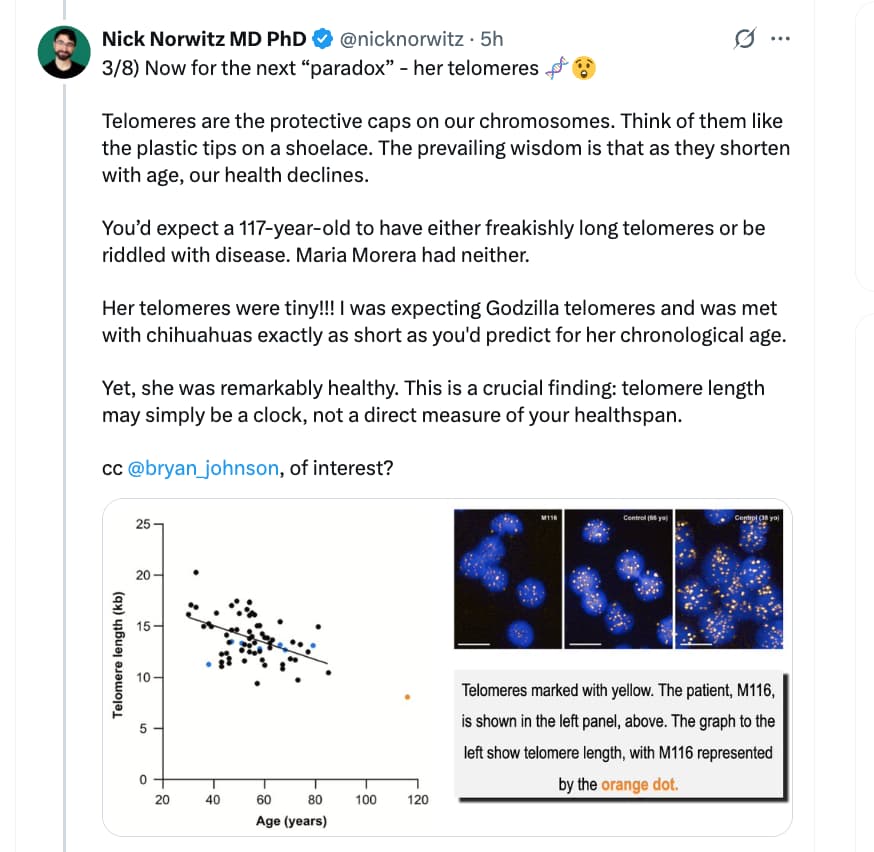
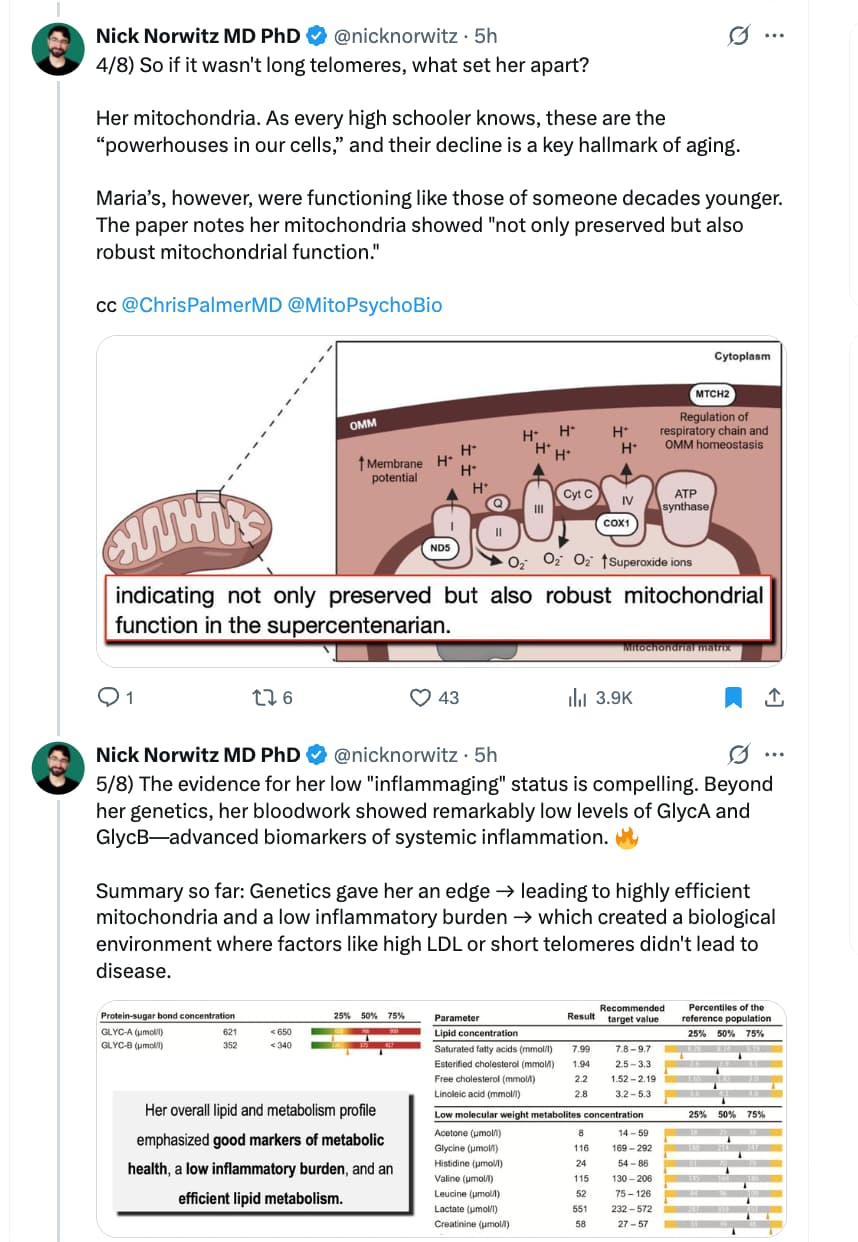
I’ve followed this thread with interest but haven’t had the time to respond until now.
Consuming probiotics doesn’t guarantee that they can get to where you want them.
Via ChatGPT…
1. Survival Through the GI Tract
- The stomach and upper small intestine present harsh conditions:
- Low pH (acidic environment in the stomach)
- Bile salts and digestive enzymes in the small intestine
- Many microorganisms get killed here, but some probiotic strains (e.g., certain Lactobacillus and Bifidobacterium) demonstrate natural resistance, while others are delivered in protective capsules or formulations designed to improve survival.
2. Evidence of Reaching the Colon
- Clinical studies have shown that some orally consumed probiotics can indeed be recovered alive from stool samples, which means they survive passage and reach the large intestine.
- Survival rates vary a lot depending on the strain, dose, and formulation — often estimated in the 1–10% range of what’s ingested.
3. Colonization vs. Transient Effects
- Most probiotics do not permanently colonize the gut. Instead, they exert effects while passing through, such as producing metabolites (like short-chain fatty acids), competing with pathogens, or modulating immune responses.
- For sustained benefits, regular intake often becomes necessary.
4. Strength & Dosing
- Because survival is partial, supplements typically contain high doses (e.g., billions of CFU) to ensure that at least some meaningful number of organisms make it to the colon.
- Different strains vary widely in effectiveness — Saccharomyces boulardii (a yeast) is quite resilient, while many bacteria need encapsulation to survive transit.
Bottom line: Yes, some orally consumed probiotics do reach the lower intestine in sufficient strength to affect the microbiome, but survival rates are limited, strain-dependent, and usually transient. The clinical effects depend heavily on the specific organism and formulation.
Bacteria with Strong Survival & Evidence
1. Lactobacillus rhamnosus GG (LGG)
- Survival: Acid- and bile-tolerant. Frequently recovered in stool.
- Effects: Reduces antibiotic-associated diarrhea, supports immune response, and may protect against some infections.
- Notes: One of the most clinically tested strains.
2. Bifidobacterium animalis subsp. lactis (BB-12, Bi-07, etc.)
- Survival: Good survival in GI tract. Detected in fecal samples.
- Effects: Improves stool consistency, supports lactose digestion, reduces risk of GI infections.
- Notes: Often combined with LGG in yogurt or capsules.
3. Lactobacillus plantarum (299v)
- Survival: Can adhere to intestinal mucosa, resisting washout.
- Effects: Reduces bloating/IBS symptoms, may support iron absorption.
- Notes: Found in fermented foods and supplements.
4. Bifidobacterium longum (various subspecies)
- Survival: Strain-dependent, but several survive well with encapsulation.
- Effects: Supports mood and cognition (“psychobiotic” effects), reduces inflammation.
- Notes: Some formulations targeted at gut-brain axis.
Yeast with Strong Survival
5. Saccharomyces boulardii CNCM I-745
- Survival: Excellent; resistant to stomach acid, bile salts, and antibiotics.
- Effects: Well-proven against antibiotic-associated diarrhea, Clostridioides difficile recurrence, and traveler’s diarrhea.
- Notes: Acts differently from bacteria — more transient, but very effective.
Important Considerations
- Formulation matters: Capsules with enteric coating or microencapsulation improve survival.
- Dose matters: Clinical trials often use 10⁹–10¹¹ CFU/day.
- Strain specificity: Benefits don’t apply across species — e.g., L. rhamnosus GG ≠ L. rhamnosus generic.
Bottom line: The strains with the best survival and consistent evidence in humans are L. rhamnosus GG, B. animalis lactis, L. plantarum 299v, B. longum, and S. boulardii .
S T R A T E G Y
I have some autoimmune issues I’ve described in the forum and have taken up approaches to address them.
Other than fecal transplants (limited in the USA) I think we have 3 available methods to affect the gut microbiome:
-
Dysbiosis: (i) Identify (sequence gut microbiome) dysbiosis (overgrowth of bad stuff), (ii) knock it down, (iii) Targeted antibiotics have helped me, iv. Maybe other medications could as well (lots of stuff down there other than bacteria that can go wrong.
-
Probiotics: Introduce probiotics to colonize favorable flora and fauna. Getting them to where one needs them presents challenges.
-
Prebiotics: Introduce prebiotics/fiber that (i) can reach the gut, (ii) ferment, and (iii) support colonization of favorable flora and fauna.
More on prebiotics:
-
Resistant starch: I currently take resistant starch (4 tbs potato starch) early in the day.
-
Inulin + psyllium: a combination of inulin (2 tbs) + psyllium seed husks (2 tbs) around 4 PM.
-
Beta-glucan: I want to add soluble beta-glucan to boost butyrate which can in turn address leaky gut associated with autoimmune conditions, although it seems that not all sources of Beta-Glucan do this. As I understand, oat and barley sourced beta glucan do, I just haven’t identified a good source.
-
Fermentation timing: The separation matters as the fibers compete for fermentation in the gut.
Thoughts | comments appreciated.