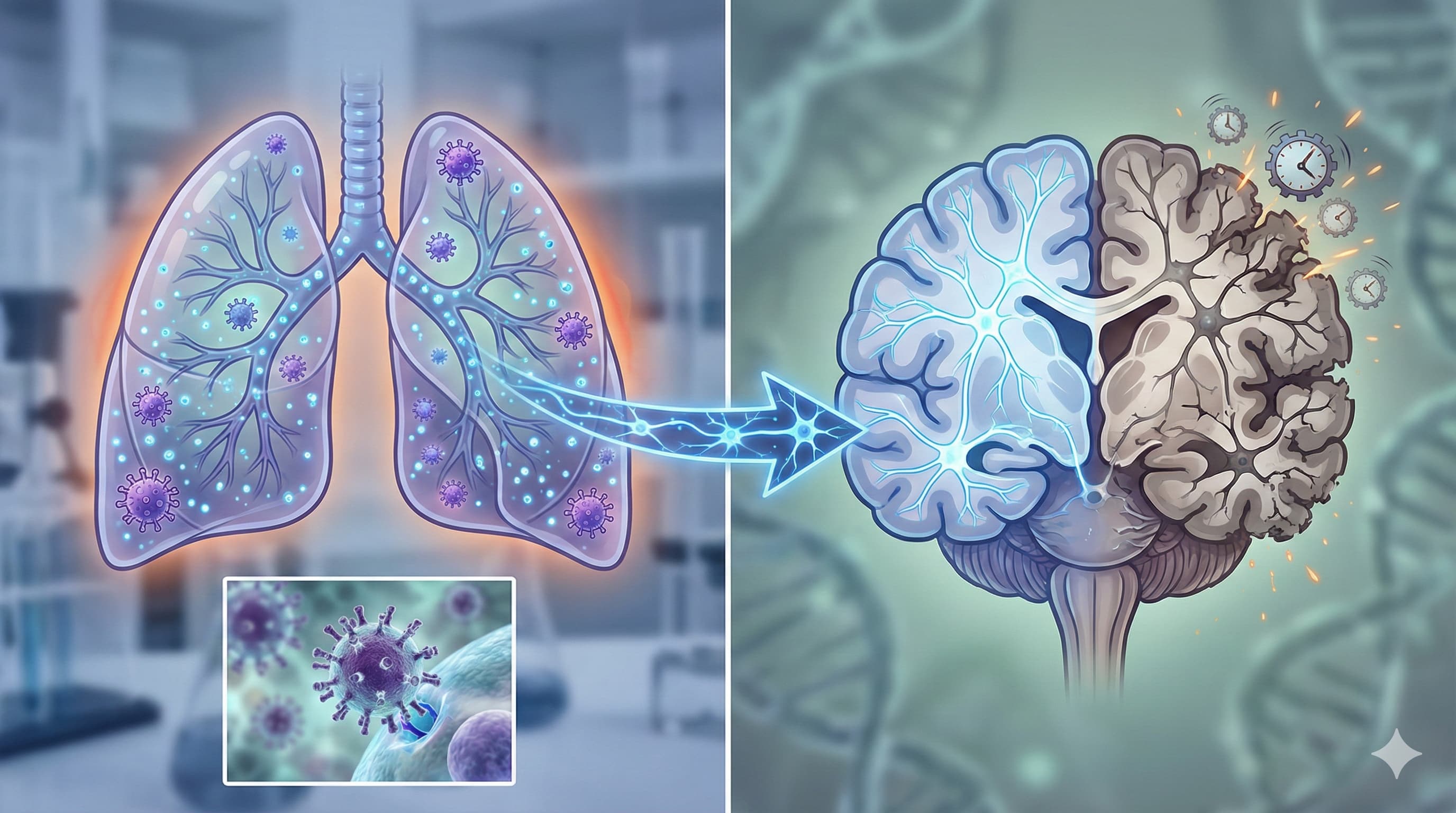
The “Long Flu” Effect: Common Infections May Irreversibly Age Your Brain
We often view the flu as a transient respiratory annoyance—a week of fever, coughing, and misery, followed by a return to normal. This study (in preprint at BioRxiv) shatters that assumption, revealing that non-neuroinvasive Influenza A pneumonia triggers a permanent “aging event” in the brain. Researchers discovered that even when the virus never enters the central nervous system, the systemic inflammation it causes drives microglia (the brain’s immune cells) into a “Disease-Associated Microglia” (DAM) state.
Most alarmingly, this effect was age-dependent. In middle-aged mice (equivalent to humans in their 40s–50s), a single severe flu infection accelerated microglial aging by the equivalent of ~30 human years, pushing them into a state typically seen only in geriatric brains. This DAM phenotype—characterized by a metabolic crash and white matter inflammation—did not resolve after recovery. The study suggests that accumulating “hits” from common respiratory infections may be a primary driver of the cognitive decline we associate with normal aging, effectively fast-forwarding the neurodegenerative clock.
Novelty & Significance
- The “Middle-Age Cliff”: Young mice recovered fully from flu. Old mice were already inflamed. Middle-aged mice suffered the most dramatic, irreversible shift. This suggests a “critical window” in mid-life where systemic insults permanently alter brain trajectory.
- Environmental Priming: Microglial depletion (using PLX3397) and repopulation failed to reset the clock. The repopulated microglia immediately adopted the aged/DAM phenotype, proving the signal comes from the aged brain environment (likely damaged white matter), not the cells themselves.
The “Real” Protocol: Metabolic & Preventive Strategy
Since the “reset” drug failed, the actionable intelligence lies in preventing the “acceleration event” and managing the metabolic fallout.
-
Priority 1: The “Vaccine Shield”
- Rationale: The study shows that viral pneumonia in middle age causes permanent neuroinflammation.
- Action: Strict adherence to annual Influenza and COVID-19 vaccination. Preventing severe pneumonia is arguably a potent anti-dementia strategy for individuals over 40.
Critical Limitations
- Sex Bias: The study used only male mice. Given that women have higher rates of autoimmune/inflammatory brain disease (like AD) and different immune responses to flu, this is a major blind spot.
- No Cognitive Correlation: While they mapped cellular changes, they did not perform behavioral tests (e.g., Morris Water Maze) to prove these cellular shifts caused actual memory loss in this specific cohort.
- Viral Specificity: It is unclear if this is specific to Influenza A or if COVID-19/RSV would trigger the exact same DAM pathways (though mechanisms overlap).
Institution: Northwestern University, USA Journal: bioRxiv (Preprint) Impact Evaluation: This manuscript is currently a preprint on bioRxiv and has not yet been peer-reviewed or assigned a Journal Impact Factor (JIF). However, the lead institutions (Northwestern, Chan Zuckerberg Biohub) and the scope of the data (multi-omics, spatial transcriptomics, human validation) suggest this is “High” impact research likely targeting top-tier journals (Nature Aging, Cell, etc).
Full open access paper: Influenza A infection accelerates disease-associated microglia formation during physiological aging
Full Gemini Analysis: https://gemini.google.com/share/4bc54a077e7c
Related Reading: Vaccines for longevity