This is why I don’t like camping.
Not recommended under 60.
@Dr.Bart I appreciate that. And while I have you, I just heard Eric Verdin mention he got a titer to see if he needed a new measles vax. I had been meaning to look into this.
I was born in 1966, and the internet says I potentially need a new MMR vax.
I assume getting a titer done would be the smart way to do this, and if you agree can you give me the correct verbiage on what I should specially ask for? I’m all ears if you have another suggestion.
You can check titers for just about everything: MMR, DTaP, COVID, etc.
DTaP is the one that needs repeating, not sure about MMR.
Critical analysis of this video’s points from my DIA ai browser: diabrowser.com
Key Points From the Video, and analysis of the credibility of it:
Sharyl Attkisson’s 23-minute report centers on former Merck virologist Stephen Krahling, who says he discovered in 1999 that the mumps component of Merck’s MMR vaccine degraded faster than the FDA allowed.
- According to Krahling, Merck responded by “overfilling” vials with extra live virus so that the product would still meet the potency floor after two years on the shelf. He argues this meant children vaccinated soon after release could receive a higher-than-tested dose, posing safety questions.
- When overfilling allegedly failed to keep potency high enough, Merck launched “Protocol 7,” a study designed—Krahling claims—to show the vaccine was still effective at lower mumps-virus levels. He contends supervisors ordered data to be altered or discarded to make the results look better.
- Krahling says he alerted the FDA in 2001 and later became a lead relator in a 2010 False Claims Act/antitrust suit accusing Merck of misrepresenting mumps efficacy to the government.
- The segment notes that, after 14 years of litigation, a federal court dismissed the whistle-blower case in 2023, ruling that the CDC already knew of potency concerns yet continued to purchase the vaccine, so no fraud against taxpayers was proven.
- Attkisson links continuing mumps outbreaks in highly vaccinated populations to the questions Krahling raises about waning potency and transparency.
Critical Evaluation Against Current Scientific Evidence
1. Potency “Overfill” and Safety
Live-virus vaccines do lose titer over time, and regulators routinely instruct manufacturers to add a buffer—“overfill”—so every dose meets the minimum potency at expiry. This practice is described in FDA biologics guidance and in publicly available package inserts; there is no evidence that the initial concentration ever exceeds the maximum level judged safe in clinical trials. Surveillance systems and controlled trials have not shown an increased rate of adverse events in children vaccinated soon after lot release.
2. Real-world Mumps Outbreaks
Multiple peer-reviewed studies and CDC reviews agree that two MMR doses provide very strong protection against measles and rubella, but mumps immunity can wane. Current U.S. estimates place two-dose effectiveness for mumps at roughly 86 % and one-dose at about 72 %. When outbreaks occur—usually in close-contact settings such as college dorms—public-health officials recommend a third dose, which quickly curbs transmission. These observations are consistent with time-dependent waning, not necessarily with a chronically sub-potent product.
3. The Whistle-blower Litigation
The Protocol 7 allegations were examined exhaustively in Krahling v. Merck. In 2023 the U.S. District Court for the Eastern District of Pennsylvania granted summary judgment to Merck, and the Third Circuit affirmed in 2024, finding that—even if some internal data were questionable—federal agencies had independent evidence of declining field effectiveness, understood the limitations, and still deemed the vaccine essential. The ruling did not declare the vaccine ineffective; it held that any misstatements were not “material” to government purchasing decisions.
4. Transparency and Labeling
Every MMR lot must meet FDA-specified upper and lower potency limits before release; results are auditable and subject to inspection. While the exact plaque-forming-unit (PFU) count in an individual child’s syringe is not printed on the label, the allowable range and test methods are publicly described in the license and package insert. Informed-consent standards require disclosure of known risks, not precise PFU figures for each lot. Major professional bodies (CDC’s ACIP in the U.S. and NACI in Canada) continue to recommend MMR without reservation.
5. Bottom Line
• Krahling’s account usefully highlights the importance of rigorous potency testing and whistle-blower protections.
• However, the best available data indicate that MMR remains both safe and highly effective; when mumps outbreaks appear, they are largely explained by waning immunity in young adults rather than by chronically sub-standard vaccine lots.
• Regulatory scrutiny continues: the FDA monitors potency, the CDC tracks effectiveness, and since 2022 a second manufacturer (GSK) offers an FDA-licensed mumps vaccine—further evidence that the agency is not shielding a single supplier.
Taken together, the scientific consensus does not support the video’s implication that U.S. children are routinely receiving unsafe or ineffective MMR doses, though it does reinforce the need for ongoing evaluation of booster strategies as immunity wanes.
We knew the potential link between infections and Alzheimer’s disease. Now Covid and Parkinson’s disease: Parkinson's disease - #934 by adssx
Interesting study here from Italy:
file:///C:/Users/User/Downloads/excli2025-8400.pdf
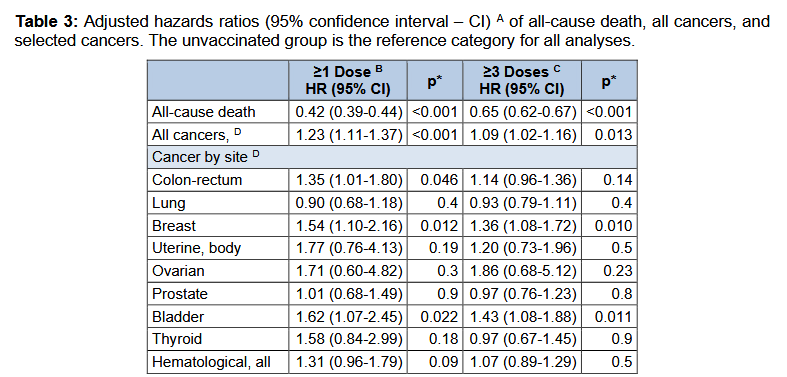
The Covid Vax apparently improves (decreases) all cause mortality for the time of the study while increasing several types of cancer.
Ovarian was really high, but not significant. I have trouble with that too.
What’s the source? (your upload didn’t work)
Try this:
Thanks. It seems to be a fairly shitty journal, which makes me question the validity of the findings. If very good research I would expect a better journal to publish it. Still, a massive reduction in all-cause death is what matters to me over 30 month. The finding for cancer hospitalization “varied by infection status, cancer site, and the minimum lag-time after vaccination” so it seems less reliable.
Scientists have previously found the vaccine appears to cut the risk of diabetes. This may be due to the hepatitis B virus – which infects the liver and spreads through blood, semen and vaginal fluids – disrupting the organ’s ability to store sugar from the blood. This could raise the risk of diabetes, where blood sugar levels are persistently too high.
But prior studies have not looked at whether the vaccine might reduce diabetes risk among a group of both immunised and non-immunised people who haven’t contracted hepatitis B, which would suggest the effect acts independently of just preventing the infection.
To explore this, Nhu-Quynh Phan at Taipei Medical University in Taiwan and her colleagues analysed the health records of more than 580,000 people living across the US, Europe, Africa, Latin America, the Middle East and the Asia-Pacific. On average, these records spanned nearly four years for each individual between 2005 and 2023.
The team found the vaccinated participants had an overall 15 per cent lower rate of diabetes – defined as them either receiving a diagnosis, having a persistently high blood sugar level or being prescribed diabetes drugs – than their unvaccinated counterparts. The vast majority of cases were type 2 diabetes, the most common form of the condition. The results will be presented at the European Association for the Study of Diabetes Annual Meeting in Vienna, Austria.
Although this is observational research, the scientists also found a dose-response effect, where the vaccinated participants with higher levels of hepatitis B-specific antibodies were less likely to develop diabetes than those with lower levels. Differences in antibody levels may be a reflection of how many vaccine doses the individual participants received, how recently they were immunised or general variation in immune responses.
Read the full story:
@Bicep , an ChatGPT analysis of that study, notice the weaknesses:
Here’s a concise, methods-focused critique of the EXCLI Journal cohort study you linked.
What the study did (in brief)
- Population-wide cohort of residents ≥11 y in Pescara, Italy, followed June 27 2021–Dec 31 2023. Exposure = COVID-19 vaccination (≥1 dose; ≥3 doses). Outcomes = all-cause mortality and first hospital admission with a cancer diagnosis (skin cancers excluded), identified via ICD-9 codes. Cox models adjusted for age, sex, selected comorbidities and recorded prior SARS-CoV-2 infection; sensitivity analyses imposed 90/180/365-day lags from vaccination to outcome. (excli.de)
- Key results: vaccinated groups had lower all-cause mortality; cancer hospitalization risk was modestly higher for “≥1 dose” at 180 days (HR≈1.23) but the association disappeared or reversed with a 365-day lag (≥3 doses HR≈0.90). Authors emphasize findings are preliminary due to healthy-vaccinee bias and unmeasured confounding. (excli.de)
Strengths
- Very large, population-level dataset with deterministic linkage across health registries. (excli.de)
- Prespecified adjustments for major clinical covariates; proportional-hazards assumptions checked; multiple lag-period sensitivity analyses. (excli.de)
- Authors openly discuss healthy-vaccinee bias and testing misclassification, appropriately tempering causal claims. (excli.de)
Main weaknesses / sources of bias
-
Outcome ≠ incidence (ascertainment bias).
“Cancer” is proxied by first hospital admission with a cancer code, not incident diagnosis. Admission practices, care-seeking, and screening intensity differ by age, sex, SES, and health behaviors—factors unevenly distributed between vaccinated and unvaccinated groups. This can inflate apparent cancer “risk” without reflecting true incidence (e.g., more screening → more admissions for surgery). The paper acknowledges higher vaccinated age/comorbidity profiles but lacks data on screening participation, primary-care use, or socioeconomic variables. (excli.de) -
Exposure handling may induce time-related bias.
Follow-up for vaccinated starts 180 days after the 1st/3rd dose; unvaccinated follow-up begins on fixed calendar dates. Vaccination status appears treated as group membership rather than a time-varying exposure, raising risk of immortal-time and misclassification bias (person-time prior to vaccination may be misattributed). The paper does not report time-updated vaccination in the Cox model. (excli.de) -
Competing risks not modeled.
Vaccination is strongly associated with lower all-cause mortality (HR≈0.42 for ≥1 dose). Lower death rates leave more vaccinated individuals alive to be hospitalized later, potentially increasing observed hospitalization counts independent of cancer biology. No Fine-Gray or other competing-risk analyses were presented. (excli.de) -
Residual confounding is substantial and mostly unaddressed.
Adjustments cover a few hospital-coded comorbidities and recorded infections. Missing are smoking, alcohol, obesity/BMI, occupational exposures, family history, medications, and socioeconomic status—key determinants for breast, colorectal, bladder, and hematologic cancers. The authors concede unmeasured confounding and healthy-vaccinee bias but cannot quantify them. (excli.de) -
Fragility to analytic choices / multiple testing.
Signals fluctuate across lags, sexes, cancer sites, and vaccine products (e.g., 180-day HR>1 for several sites; 365-day lag attenuates or reverses). No correction for multiple comparisons is reported despite many subgroup and sensitivity analyses, increasing false-positive risk. (excli.de) -
Infection status misclassification.
“Prior SARS-CoV-2 infection” relies on recorded tests only, in a context where testing policies changed and many infections went unrecorded. Stratified results differ sharply by recorded infection, which the authors warn should be interpreted cautiously. (excli.de) -
Generalizability and construct validity.
Single-province setting; outcome is hospital admission, not registry-confirmed incidence or stage. Skin cancers excluded; stage, treatment intent, and pathology data unavailable. Manufacturer-stratified results are presented, but product groups differ in age/indication mixes; confounding by indication likely. (excli.de) -
Mechanistic citations lean on speculative literature.
The discussion cites hypothesis papers and preprints proposing oncogenic mechanisms of vaccination (e.g., mRNA/LNP effects, DNA contamination) and explicitly says they “need validation.” Including these does not strengthen causal inference from the current observational signal. (excli.de)
How the analysis could be strengthened
- Use time-varying vaccination and infection status (target-trial emulation), with calendar-time and time-since-vaccination terms to address dynamic pandemic conditions.
- Model competing risks (death) and present absolute risks and risk differences alongside HRs.
- Link to cancer registry data (date of diagnosis, histology, stage) and screening records(mammography/colonoscopy) to reduce misclassification and screening bias.
- Include negative-control outcomes/exposures to detect residual bias; consider self-controlled designs for short-term diagnostic surges.
- Adjust or report for multiple testing (e.g., FDR) across numerous site-/sex-/product-specific analyses.
Bottom line
The study’s own sensitivity analyses and caveats point to instability of associations for cancer hospitalization and major susceptibility to residual and time-related biases. As designed, it does not establish a causal link between vaccination and cancer risk (in either direction). The clearest signal—lower all-cause mortality among the vaccinated—is itself likely influenced by healthy-vaccinee and other unmeasured factors the authors acknowledge. Overall, the paper is hypothesis-generating and underlines the need for registry-based incidence analyses with rigorous time-varying and competing-risk methods before drawing conclusions about cancer risk. (excli.de)
How to get a coronavirus vaccine and who’s eligible amid limited access
Pharmacies and state health departments are scrambling to adapt to the confusion over a vaccine that is no longer broadly recommended.
Cast a wide net
Doctors and patient advocates stress you might need to make a lot of phone calls, book multiple online appointments with different providers and scour the internet for reputable advice from medical organizations to secure a shot.
Advocates report that a CDC website intended to help patients find vaccine providers has not been working. But other websites, such as EasyVax (run by pharmaceutical company GSK) and VaccineInformation, feature other useful online tools for finding pharmacies or federally funded clinics that might offer coronavirus vaccines.
Read the full story: How to get a coronavirus vaccine and who’s eligible amid limited access (Washington Post)
This will be interesting
It is interesting. I wish there was a way to get independent 3rd party data on efficacy on things like this. I don’t trust data on efficacy on this coming out of the Russian govt. but I don’t see any other 3rd parties doing any validation testing (I’d like the same thing on the Chinese 'flozins" that are showing telomere benefits).
Most likely BS, unfortunately.
This is different, it’s probably just a class benefit.
A markedly younger age was observed among the unvaccinated (mean 45.1±19.7 years), compared to the subjects who re-ceived at least three vaccine dose (mean 53.0±20.0 years; p<0.001; Student’s t-test for unpaired samples) or at least one dose (mean 50.2±20.5 years; p<0.001).
Would that be a factor?
Finally, after stratifying by vaccine type, all except mRNA-1273 were positively associated with the overall cancer hospital admissions (Table S2).
Looks like Moderna is in the clear. All my COVID vaccinations (>3). were Moderna shots.
the hazard of being hospitalized for cancer was higher in individuals that received at least one vaccine dose, com-pared to the unvaccinated, but did not in-crease when the analyses were restricted to those exposed to at least three doses. Such an apparent lack of dose-response could either challenge the hypothesis of oncogenesis, suggesting that the observed associations are to be attributed to unmeasured, confounding factors, or just indicate that a single dose could be sufficient to trigger the potential tumorigenic action.
Table 3 clearly shows that. Looks like more is better.
I’m not nearly as good at reading studies as you are Juan. I try reading it, then usually try to find somebody smart and unbiased to splain it to me. John Campbell told people for the first year or so to go get the shot, then changed his mind and is now nearly always much more cautious. He has a phd in nursing:
I usually watch him at 1.5x speed as he is a slow talker.
I worry about the immune system damage. My daughter got the cancer after getting the jab. Also I’m pretty sure they’ve proven DNA of the SV40 is in there. It is possible they know what they’re doing. It’s also possible they’re trying to thin the herd (for our own good of course). With brilliant people on both sides, my position is that I’m safer not taking vaccines.
I can’t believe I let them give half of my kids a Hep B vaccine on their first day of life. There was no reason to do this at all. They’ve gone too far, way to bold betting our health. Even with the howling against him, I still like the guy that sounds like he’s talking through a fan.