Interesting study here from Italy:
file:///C:/Users/User/Downloads/excli2025-8400.pdf
The Covid Vax apparently improves (decreases) all cause mortality for the time of the study while increasing several types of cancer.
Ovarian was really high, but not significant. I have trouble with that too.
What’s the source? (your upload didn’t work)
Try this:
Thanks. It seems to be a fairly shitty journal, which makes me question the validity of the findings. If very good research I would expect a better journal to publish it. Still, a massive reduction in all-cause death is what matters to me over 30 month. The finding for cancer hospitalization “varied by infection status, cancer site, and the minimum lag-time after vaccination” so it seems less reliable.
Scientists have previously found the vaccine appears to cut the risk of diabetes. This may be due to the hepatitis B virus – which infects the liver and spreads through blood, semen and vaginal fluids – disrupting the organ’s ability to store sugar from the blood. This could raise the risk of diabetes, where blood sugar levels are persistently too high.
But prior studies have not looked at whether the vaccine might reduce diabetes risk among a group of both immunised and non-immunised people who haven’t contracted hepatitis B, which would suggest the effect acts independently of just preventing the infection.
To explore this, Nhu-Quynh Phan at Taipei Medical University in Taiwan and her colleagues analysed the health records of more than 580,000 people living across the US, Europe, Africa, Latin America, the Middle East and the Asia-Pacific. On average, these records spanned nearly four years for each individual between 2005 and 2023.
The team found the vaccinated participants had an overall 15 per cent lower rate of diabetes – defined as them either receiving a diagnosis, having a persistently high blood sugar level or being prescribed diabetes drugs – than their unvaccinated counterparts. The vast majority of cases were type 2 diabetes, the most common form of the condition. The results will be presented at the European Association for the Study of Diabetes Annual Meeting in Vienna, Austria.
Although this is observational research, the scientists also found a dose-response effect, where the vaccinated participants with higher levels of hepatitis B-specific antibodies were less likely to develop diabetes than those with lower levels. Differences in antibody levels may be a reflection of how many vaccine doses the individual participants received, how recently they were immunised or general variation in immune responses.
Read the full story:
@Bicep , an ChatGPT analysis of that study, notice the weaknesses:
Here’s a concise, methods-focused critique of the EXCLI Journal cohort study you linked.
What the study did (in brief)
- Population-wide cohort of residents ≥11 y in Pescara, Italy, followed June 27 2021–Dec 31 2023. Exposure = COVID-19 vaccination (≥1 dose; ≥3 doses). Outcomes = all-cause mortality and first hospital admission with a cancer diagnosis (skin cancers excluded), identified via ICD-9 codes. Cox models adjusted for age, sex, selected comorbidities and recorded prior SARS-CoV-2 infection; sensitivity analyses imposed 90/180/365-day lags from vaccination to outcome. (excli.de)
- Key results: vaccinated groups had lower all-cause mortality; cancer hospitalization risk was modestly higher for “≥1 dose” at 180 days (HR≈1.23) but the association disappeared or reversed with a 365-day lag (≥3 doses HR≈0.90). Authors emphasize findings are preliminary due to healthy-vaccinee bias and unmeasured confounding. (excli.de)
Strengths
- Very large, population-level dataset with deterministic linkage across health registries. (excli.de)
- Prespecified adjustments for major clinical covariates; proportional-hazards assumptions checked; multiple lag-period sensitivity analyses. (excli.de)
- Authors openly discuss healthy-vaccinee bias and testing misclassification, appropriately tempering causal claims. (excli.de)
Main weaknesses / sources of bias
-
Outcome ≠ incidence (ascertainment bias).
“Cancer” is proxied by first hospital admission with a cancer code, not incident diagnosis. Admission practices, care-seeking, and screening intensity differ by age, sex, SES, and health behaviors—factors unevenly distributed between vaccinated and unvaccinated groups. This can inflate apparent cancer “risk” without reflecting true incidence (e.g., more screening → more admissions for surgery). The paper acknowledges higher vaccinated age/comorbidity profiles but lacks data on screening participation, primary-care use, or socioeconomic variables. (excli.de) -
Exposure handling may induce time-related bias.
Follow-up for vaccinated starts 180 days after the 1st/3rd dose; unvaccinated follow-up begins on fixed calendar dates. Vaccination status appears treated as group membership rather than a time-varying exposure, raising risk of immortal-time and misclassification bias (person-time prior to vaccination may be misattributed). The paper does not report time-updated vaccination in the Cox model. (excli.de) -
Competing risks not modeled.
Vaccination is strongly associated with lower all-cause mortality (HR≈0.42 for ≥1 dose). Lower death rates leave more vaccinated individuals alive to be hospitalized later, potentially increasing observed hospitalization counts independent of cancer biology. No Fine-Gray or other competing-risk analyses were presented. (excli.de) -
Residual confounding is substantial and mostly unaddressed.
Adjustments cover a few hospital-coded comorbidities and recorded infections. Missing are smoking, alcohol, obesity/BMI, occupational exposures, family history, medications, and socioeconomic status—key determinants for breast, colorectal, bladder, and hematologic cancers. The authors concede unmeasured confounding and healthy-vaccinee bias but cannot quantify them. (excli.de) -
Fragility to analytic choices / multiple testing.
Signals fluctuate across lags, sexes, cancer sites, and vaccine products (e.g., 180-day HR>1 for several sites; 365-day lag attenuates or reverses). No correction for multiple comparisons is reported despite many subgroup and sensitivity analyses, increasing false-positive risk. (excli.de) -
Infection status misclassification.
“Prior SARS-CoV-2 infection” relies on recorded tests only, in a context where testing policies changed and many infections went unrecorded. Stratified results differ sharply by recorded infection, which the authors warn should be interpreted cautiously. (excli.de) -
Generalizability and construct validity.
Single-province setting; outcome is hospital admission, not registry-confirmed incidence or stage. Skin cancers excluded; stage, treatment intent, and pathology data unavailable. Manufacturer-stratified results are presented, but product groups differ in age/indication mixes; confounding by indication likely. (excli.de) -
Mechanistic citations lean on speculative literature.
The discussion cites hypothesis papers and preprints proposing oncogenic mechanisms of vaccination (e.g., mRNA/LNP effects, DNA contamination) and explicitly says they “need validation.” Including these does not strengthen causal inference from the current observational signal. (excli.de)
How the analysis could be strengthened
- Use time-varying vaccination and infection status (target-trial emulation), with calendar-time and time-since-vaccination terms to address dynamic pandemic conditions.
- Model competing risks (death) and present absolute risks and risk differences alongside HRs.
- Link to cancer registry data (date of diagnosis, histology, stage) and screening records(mammography/colonoscopy) to reduce misclassification and screening bias.
- Include negative-control outcomes/exposures to detect residual bias; consider self-controlled designs for short-term diagnostic surges.
- Adjust or report for multiple testing (e.g., FDR) across numerous site-/sex-/product-specific analyses.
Bottom line
The study’s own sensitivity analyses and caveats point to instability of associations for cancer hospitalization and major susceptibility to residual and time-related biases. As designed, it does not establish a causal link between vaccination and cancer risk (in either direction). The clearest signal—lower all-cause mortality among the vaccinated—is itself likely influenced by healthy-vaccinee and other unmeasured factors the authors acknowledge. Overall, the paper is hypothesis-generating and underlines the need for registry-based incidence analyses with rigorous time-varying and competing-risk methods before drawing conclusions about cancer risk. (excli.de)
How to get a coronavirus vaccine and who’s eligible amid limited access
Pharmacies and state health departments are scrambling to adapt to the confusion over a vaccine that is no longer broadly recommended.
Cast a wide net
Doctors and patient advocates stress you might need to make a lot of phone calls, book multiple online appointments with different providers and scour the internet for reputable advice from medical organizations to secure a shot.
Advocates report that a CDC website intended to help patients find vaccine providers has not been working. But other websites, such as EasyVax (run by pharmaceutical company GSK) and VaccineInformation, feature other useful online tools for finding pharmacies or federally funded clinics that might offer coronavirus vaccines.
Read the full story: How to get a coronavirus vaccine and who’s eligible amid limited access (Washington Post)
This will be interesting
It is interesting. I wish there was a way to get independent 3rd party data on efficacy on things like this. I don’t trust data on efficacy on this coming out of the Russian govt. but I don’t see any other 3rd parties doing any validation testing (I’d like the same thing on the Chinese 'flozins" that are showing telomere benefits).
Most likely BS, unfortunately.
This is different, it’s probably just a class benefit.
A markedly younger age was observed among the unvaccinated (mean 45.1±19.7 years), compared to the subjects who re-ceived at least three vaccine dose (mean 53.0±20.0 years; p<0.001; Student’s t-test for unpaired samples) or at least one dose (mean 50.2±20.5 years; p<0.001).
Would that be a factor?
Finally, after stratifying by vaccine type, all except mRNA-1273 were positively associated with the overall cancer hospital admissions (Table S2).
Looks like Moderna is in the clear. All my COVID vaccinations (>3). were Moderna shots.
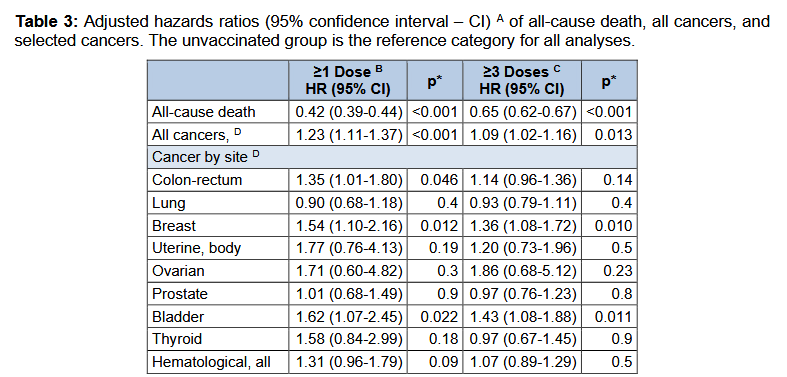
the hazard of being hospitalized for cancer was higher in individuals that received at least one vaccine dose, com-pared to the unvaccinated, but did not in-crease when the analyses were restricted to those exposed to at least three doses. Such an apparent lack of dose-response could either challenge the hypothesis of oncogenesis, suggesting that the observed associations are to be attributed to unmeasured, confounding factors, or just indicate that a single dose could be sufficient to trigger the potential tumorigenic action.
Table 3 clearly shows that. Looks like more is better.
I’m not nearly as good at reading studies as you are Juan. I try reading it, then usually try to find somebody smart and unbiased to splain it to me. John Campbell told people for the first year or so to go get the shot, then changed his mind and is now nearly always much more cautious. He has a phd in nursing:
I usually watch him at 1.5x speed as he is a slow talker.
I worry about the immune system damage. My daughter got the cancer after getting the jab. Also I’m pretty sure they’ve proven DNA of the SV40 is in there. It is possible they know what they’re doing. It’s also possible they’re trying to thin the herd (for our own good of course). With brilliant people on both sides, my position is that I’m safer not taking vaccines.
I can’t believe I let them give half of my kids a Hep B vaccine on their first day of life. There was no reason to do this at all. They’ve gone too far, way to bold betting our health. Even with the howling against him, I still like the guy that sounds like he’s talking through a fan.
Here is the schedule of vaccinations for Hong Kong children. We have the longest lifespans in the world. Obviously vaccines did not hurt.
Another interesting fact. Hong Kong children have an autism rate of 1.4% while American children have a rate of 3.2%. Why do American children have more than 2X the autism rate? The UK and Canada are also at about 2%. Why are Americans so unlucky when they have a similar vaccination schedule? Might it not be vaccines???

There is no credible evidence for vaccines causing autism
It just might be the ultra processed food in the maternal diet.
A western dietary pattern during pregnancy is associated with neurodevelopmental disorders in childhood and adolescence | Nature Metabolism
It is quite possible that this links to the mtDNA germline issue.
I’m really sorry to hear about your daughter. However, I think the “damage to immune system” from vaccines is over-rated. If you actually get a viral infection, that virus enters your cells, multiplies, escapes, spreads etc. The number of viral copies increases over time until you start to deal with it (or die). Many viruses alter your DNA by integrating into the genome (like how HPV or HepB cause cancer). A vaccine exposes your body to a small amount of antigenic material, which does not replicate, does not spread anywhere near as much.
If we take HepB for example (which I understand because I received this vaccine, and did mandatory training about it); a common vaccine dose is 10µg for kids and 20µg for adults. That’s a single protein antigen (HBsAg). However, if you actually get HepB, your HBsAg level in the blood is measured in milligrams per ml - i.e. there are literally grams of it in your body - 1,000x more than the vaccine. Plus of course all the other viral protein aside from HBsAg. And for a vaccinated person, they will generally be negative for HBsAg in the blood even after exposure, because antibodies neutralise it. So as a net, vaccination exposes you to far less viral proteins, and should be less stressful for the immune system.
I don’t disagree with you, but surely that question is far too complex to try answer here, let alone evaluate whether vaccines are related. There are genetic differences, cultural differences, healthcare system differences etc. In the US now, teachers and everybody are hyper-aware and doctors are relatively quick to diagnose autism/ADHD etc. When I was a kid, there were definitely kids in my class who were odd, troublemakers etc. Today they’d all be diagnosed with something.
One theory I’ve heard is maternal and paternal age correlates with neurodevelopment disorders. But I am pretty sure HK has generally older people having kids than the US, which is interesting.
I think some of the neurodevelopment disorders have links to mitochondrial issues and those are associated with the mtDNA germline which is a multigenerational, but maternal only issue as mtDNA is derived only from the mother and not the father.
HPV vaccination was associated with significant reductions in the risk of hypopharyngeal and laryngeal carcinomas (8-year HR: 0.19; 95 % CI: 0.057–0.631; p = 0.0025; 20-year HR: 0.227; 95 % CI: 0.067–0.764; p = 0.0092) and leukemia (8-year HR: 0.461; p = 0.0035; 20-year HR: 0.443; p = 0.0019). No significant protection was observed for rectal, anal, oral cavity, or prostate cancers. All-cause mortality was reduced by nearly half among vaccinated individuals (8-year HR: 0.543; 20-year HR: 0.536; both p < 0.0001). Beyond epithelial malignancies, HPV vaccination may confer systemic cancer protection, particularly in hematologic and potentially neuronal tissues. These findings suggest a broader biological impact of HPV vaccination than previously recognized and underscore the need for mechanistic studies investigating HPV’s oncogenic pathways. If validated, these results could prompt the expansion of vaccination strategies to encompass broader indications and wider population coverage.
HPV vaccination and malignancy risks beyond cervical cancer: A retrospective global cohort study