@desertshores You are way ahead of most of us.
This is very interesting and tolerance to mTOR inhibition is worth considering. Why are we dosing weekly or even biweekly? Is there data to support that?
There may actually be data to question it.
Here’s the Matt study showing great results in mice giving a brief duration of drug and lasting a long time
And here’s a recent look at Drosophila showing a long memory of pulsed mTOR inhibition on the gut
I know when I first started rapamycin 5 years ago I discussed it with Alan Green for several hours . He basically guessed at the dose and dosing schedule. It was gutsy and worked out, but it was a guess.
I also feel that it may be weaker now but rather than keep upping the dose we should be pulsing it to prevent tolerance.
The studies you referenced MAC should be considered.
Maybe we should be taking a fairly hefty dose every 3-6 months.
People will argue that tolerance is only in chronic users, but aren’t we chronic when we take a drug with a long half life regularly over many years? Perhaps pulsing it less frequently would eliminate the glucose, lipid, and other concerns that may have.
It’s probably worth a post of its own.
Because we dose for longevity using PFA/WAG/SWAG!
I took a look at this study from ITP lab.
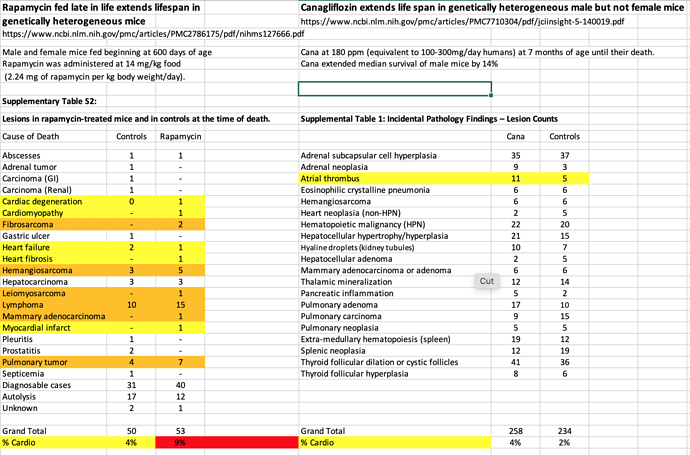
Canagliflozin extends life span in genetically heterogeneous male but not female mice
“Cana at 180 ppm (equivalent to 100-300mg/day humans) at 7 months of age until their death. Cana extended median survival of male mice by 14%” (This sexual dimorphism is very strange causing me to question their postulated theories on mortality reduction. Rapamycin is superior in that it delivers enhanced longevity in female mice too)
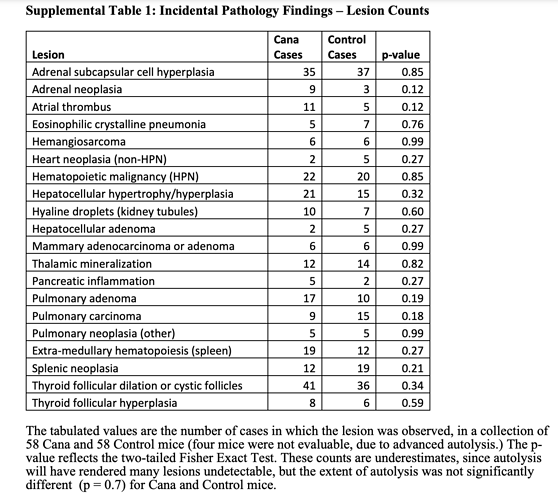
Taking a look at pathology, 90%+ cancer neoplasms.
And compared to Rapamycin study, looking to see if any different signal re cardio deaths. Seems like perhaps less cardio mediated deaths, but likely dosen’t reach significance.
Although the study authors state CANA and Rapamycin pathology outcomes similar.
“Terminal (end-of-life) necropsies for each of these 4 drugs (including Rapamycin) including the data on Cana shown here, lead to the same conclusion: the spectrum of lethal and nonlethal
late-life illnesses is not changed in quality or severity, even though the treated mice are older at time of death. Although this conclusion is limited by the low statistical power of small case series, the implication is that the drugs extend healthy life span, i.e., produce longer life span by delaying the onset or progression of the diseases most likely to lead to death or terminal morbidity, most of which are neoplastic disease in UM-HET3 and most other mouse stocks”
mTOR is not measured for this study re explaining life extension.
“These observations thus suggest strongly that the life span benefits of each drug, and presumably the other benefits already shown in acarbose-treated mice, are a consequence of lower maximal postprandial glucose levels. The basis for these protective effects is not completely understood, and may not directly reflect the effects of Cana on glucose homeostasis. Hypothetically, Cana might reduce mortality associated with neoplasia in mice by modulating glucose metabolism in tumor cells. The metabolic requirements of rapidly dividing cancer cells are quite distinct from those of normal cells, most of which are ordinarily nonmitotic”
Although they did mention mTOR1 referencing another study.
“Cana suppressed hepatic TORC1 signaling, while increasing AMPK activity in this
tissue; both effects are associated with life span extension in mice and other organisms (47)”
Great question! Maybe confirmation basis…trying to mimic chronic mice elevated dosing but avoiding the potent “human intolerable” side effects? But is this tradeoff = longevity??
If it’s “truly” mTOR driven, then we need to be depressing mTOR at least say 30% like the long lived mice?
I agree, indeed yes we are. But it’s the dose and mTOR inhibition that’s fundamentally driving the chronic administration resistance. These cohorts are just trying to live another day…we’re gunning for DECADE+
Probably, but would it deliver sufficient mTOR reduction and lifespan extension?
TOO MANY UNKNOWNS FOR HUMAN CLINICAL TRANSLATION
But we aren’t even measuring mTOR, so how are we to guide DOSING intervention??
[quote=“MAC, post:385, topic:1434”]
“But we aren’t even measuring mTOR, so how are we to guide DOSING intervention?”
[/quote],
Correct, measure mTORC1, as I have posted on another thread, link below.
Know someone with a PET scanner?
Attached is a copy of the study.
truillet2016.pdf (1.2 MB)
If you consider the Kaeberlein study, he got a 60% lifespan extension from the 3 month transient dosing. The mTOR inhibition obviously didn’t last, but the ultimate effects of the inhibit clearly lasted.
This is the longest lifespan extension that I’ve yet seen.
It also yielded some impressive healthspan results and wasn’t related to postprandial glucose levels as far as I can tell.
So why aren’t we doing this? It’s still not clear to me.
It would be informative if we got Matt’s input on his study.
I’m not sure that measuring mTOR would explain Matt’s results or help us. During the 3 month treatment it’s safe to assume that TO
R is inhibited, but after stopping rapamycin TOR levels should quickly rebound, but the effects of the inhibition lasted for the rest of their lives apparently.
Someone want to do the extrapolation to human?
You introduced just another dart board.
Indeed impressive lifespan extension, but 90 days is still a LONG dosing in mice lifespan. “Rapamycin injection at 8 mg/kg/day for 3 months”. That’s equivalent to 47 mg/day in humans. Have fun with the side effects of that. Recent Phase 2 clinical trials with Everolimus at 10 mg/day…40% drop out rate, massive side effects.
“We also define a dose in female mice that does not extend lifespan, but is associated with a striking shift in cancer prevalence toward aggressive hematopoietic cancers and away from non-hematopoietic malignancies” That’s just plain misogynistic vs chronic rapamycin dosing, where both sexes saw lifespan extension. That’s a huge discrepancy for human translation.
“Additionally, an uncommon variant of lymphoma with plasmacytoid morphology affected 4 out of 16 mice examined in the rapamycin group and no vehicle treated mice. In contrast, the incidence of non-hematopoietic neoplasms was dramatically decreased in the rapamycin group. When data from both sexes are pooled together, daily injection of 8 mg/kg rapamycin for three months resulted in a nonsignificant (p=0.16) increase in life expectancy of 23%”
“During the three-month treatment period, we noted a significant decline in body weight of male
mice receiving rapamycin injections relative to vehicle treated controls, although food intake remained similar during the treatment. Decreased body weight persisted for several weeks following cessation of treatment”
Seems to me this is a VERY important indicator of mTOR efficacy and as good as any parameter for human translation? And a consistent finding in mice studies. In the seminal cancer/GFJ study, only 11% of patients reported weight loss, although several other more concerning side effects.
I respectfully disagree. “safe to assume TOR is inhibited”? Really, and by HOW MUCH? We now know the degree of mTOR inhibition is a very sensitive and critical metric to health outcome and possible mTOR uncoupling, cancer proliferation cessation. Decrease mTOR 10%, 30%, 50%? And Rapamycin imparts different mTOR in different tissues…which tissues are most important for outcome, and how much mTOR reduction? Liver, kidney, heart, thymus, brain?
Again, you have NO idea what happened to mTOR. It was NEVER measured. A glaring weakness in this study. We know from human studies, side effects from Rapamycin quickly resolved after stopping Rapamycin dosing.
Several assumptions without the data to support.
Tell me how you are going to translate this work vs chronic rapamycin treatment studies to humans? What human dosing protocol, for what primary treatment cmax/AUC/trough/duration targets?
This is exactly why we find ourselves in this place of throwing darts…we’re not carefully doing enough dosing protocols/mTOR/biomarker/tissue/health outcome work to do a better job at human translation.
Granted it’s not an easy translation to humans but the main point remains, the effects of mTOR inhibition seem to last long after mTOR is restored. We don’t know how long the dosing would have to be or what dose, but we don’t know much about weekly dosing either. It’s all guess work but the evidence for “ memory” of mTOR inhibition is growing with the Drosophila study and it makes you wonder if we should be doing something like 3 months on and 3 months off.
Blood levels of mTOR are fine but are we going to get tissue levels of various organs ?
I do like the idea of weight loss as a biomarker. Seems that rapamycin facilitates weight loss more than directly cause it. Of course, you’re not going to continuously lose weight, but it may be a signal if you start regaining for no apparent reason.
CAC only tells you your calcified plaque load. Calcified plaque does cause heart attacks, soft plaque does. Taking statins increase the density of calcified plaque which is a good thing but will result in a higher CAC score. A CT Angiogram measures soft plaque but many cardiologists don’t bother with this. Peter Attia is a big proponent of the CTA test to really see what’s going on.
Sigh. Flies now?
This is for the researchers to drill to learn the fundamental pathways, and then translate to humans. Another member posted about an emerging pan mTOR measurement from blood. For example, if liver biomarkers are extremely correlated to mTOR and longevity, then we can measure these in humans as a biomarker. Etc. This is fundamental translation science.
Fundamental laws of physics and science do not bend to money, time, practical limits and obstacles, only humans do.
Good point, but from a risk stratification point of view (and the clinical data re CAC and future risk is VERY strong), it’s a good starting point…having a CAC score of zero vs 300 puts a dart on the board.
Soft plaque is important but multiple studies demonstrate the excellent prognostic value of the CAC as it relates to cardiovascular risk. It’s also inexpensive, easy, and low radiation.
Yes, me too. If every rapamycin/mice/longevity study consistently shows weight loss (and we do understand the metabolic pathways perturbation), why wouldn’t this be a translatable marker?
Would take careful control of diet/lifestyle as n=1 not to confound a rapamycin dosing protocol experiment.
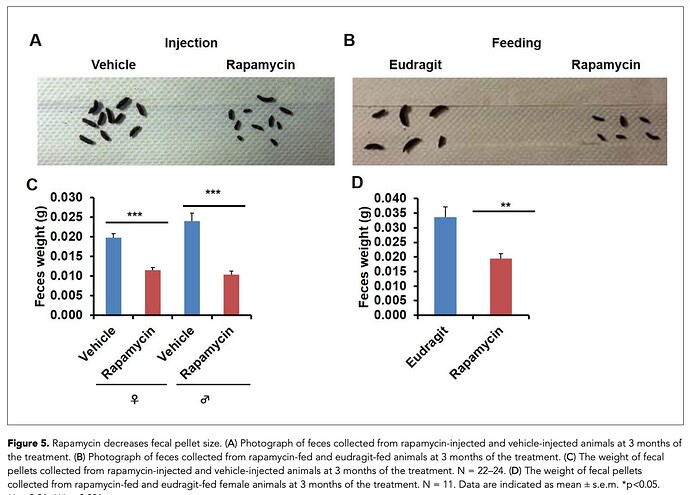
How about something a little cruder, yet still equally translatable. And we can all measure this.
“In the course of routine animal husbandry, we noted that mice treated with either rapamycin regimen produced consistently smaller fecal pellets than age-matched controls. This difference was present in both dry and freshly excreted feces indicating that water content is not a major factor affecting feces size. In addition, feces size was affected independently of the method of rapamycin delivery and the effect was persistent after cessation of treatment.”
Has anyone noticed this?
A recent article on yet another benefit. A fib is a big deal and I don’t see why the risk reduction would be limited to diabetics.
Is cost a huge issue? Is getting up frequently to urinate significant?
Very interesting but a little odd in their definitions. They call brisk walking moderate exercise- fair enough- but then refer to gardening as vigorous.
If you look at cultures that are long lived you see that they’re not exactly exercising, but tend to be very active throughout the day, and certainly not sedentary.