My Mother passed 17 days short of her 97th birthday. She was in a assisted living facility and my sister was there that morning and said
Mom got up ate just a few bites of oat meal and said I need to lay
down, she did and less that a hour later she passed. The only real
medical problem she has was rheumatoid arthritis. My sister said
the Dr from the facility said “looks like she passed from old age”
The death cert said heart failure.
I’ve read a lot of papers on longevity and centenarians, there’s no particular lifestyle and biomarker panel that is definitively centenarian, aside from the obvious low hanging fruit.
Some recent large GWAS implicate superior genetics, which has been theorized for a very long time. These studies elucidate a common sub thread…genes that reduce/delay cardiovascular disease.
Review and meta-analysis of genetic polymorphisms associated with exceptional human longevity
Results: Five polymorphisms, ACE rs4340, APOE ε2/3/4, FOXO3A rs2802292, KLOTHO KL-VS and IL6 rs1800795 were significantly associated with exceptional longevity, with the pooled effect sizes (odds ratios) ranging from 0.42 (APOE ε4) to 1.45 (FOXO3A males).
Conclusion: In general, the observed modest effect sizes of the significant variants suggest many genes of small influence play a role in exceptional longevity, which is consistent with results for other polygenic traits. Our results also suggest that genes related to cardiovascular health may be implicated in exceptional longevity."
Whole-genome sequencing analysis of semi-supercentenarians
The results showed that 105+/110+ are characterized by a peculiar genetic background associated with efficient DNA repair mechanisms, as evidenced by both germline data (common and rare variants) and somatic mutations patterns (lower mutation load if compared to younger healthy controls). Results were replicated in a second independent cohort of 333 Italian centenarians and 358 geographically matched controls. The genetics of 105+/110+ identified DNA repair and clonal haematopoiesis as crucial players for healthy aging and for the protection from cardiovascular events."
Distribution of 54 polygenic risk scores for common diseases in long lived individuals (ELLI) and their offspring
"ELLI have a similar burden of genetic disease risk as the general population for most traits but have a significantly lower genetic risk of AD and CAD"
Identification of cardiovascular health gene variants related to longevity in a Chinese population
“From a meta-analysis of venous thrombosis patients, we unexpectedly found that rs7586970 T is associated with both longevity and protection against vascular disease”
Human longevity: 25 genetic loci associated in 389,166 UK biobank participants
“For combined mothers’ and fathers’ attained age, 10 loci were associated including 8 previously identified for traits including survival, Alzheimer’s and cardiovascular disease. The results
suggest that human longevity is highly polygenic with prominent roles for loci likely involved in cellular senescence and inflammation, plus lipid metabolism and cardiovascular conditions.”
Can’t we just regularly donate blood and be done with this iron issue ?
So…unless you’ve won the genetic lottery, if you want to improve your centenarian odds, do EVERYTHING possible to preserve your vascular health. It likely has the biggest impact on your longevity.
Senescence and chronic inflammation are always popping up. Cancer is pretty much tied with CVD as causing death.
If you eliminate either of them entirely it seems to yield a life extension of about 3%.
Genetics certainly seems to play a very significant role in reaching 100+. We don’t though to what degree rapamycin will help. Maybe a great deal.
When a patient dies we’re obligated to put something down as cause of death. Often, we don’t really know.
Chief koolaid drinker! I’m on the bandwagon too, but so many unknowns. I certainly do NOT consider it a panacea in my overall interventions stack. If it helps great, but not loosing focus on maintaining a super healthy lifestyle into old age.
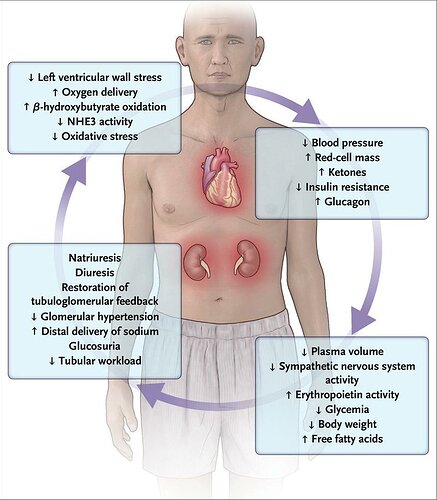
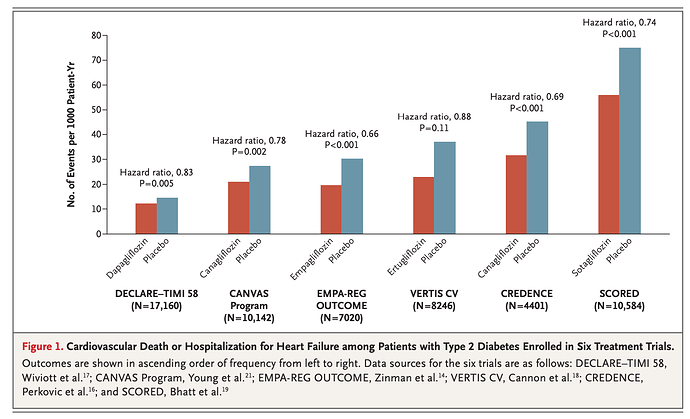
Seems like SGLT2 inhibitors might be a good match with rapamycin:
Gliflozins — sodium–glucose cotransporter 2 inhibitors — lower blood glucose and glycated hemoglobin in patients with type 2 diabetes without causing hypoglycemia. The agents also improve cardiac function in patients who have heart failure with or without type 2 diabetes and improve renal function, with few adverse effects.
Peter just came out with a good podcast about Lp(a)
Very interesting but this is becoming a case where too much information is similar to no information. Patients are confused and I guarantee you that primary care providers are confused. So far we have:
LDL
HDL
Triglyceride/HDL
ApoB
ApoA-1
ApoB/ ApoA-1
LpA
OxLDL
Total cholesterol
Now maybe it’s valuable to do all of the above, but from a practical standpoint maybe we assess the patient as a whole looking at all risk factors, and then get a coronary calcium score if deemed necessary.
If the score is high then maybe a cardiologist can sort out all of the myriad lipid tests.
Had a look through this paper.
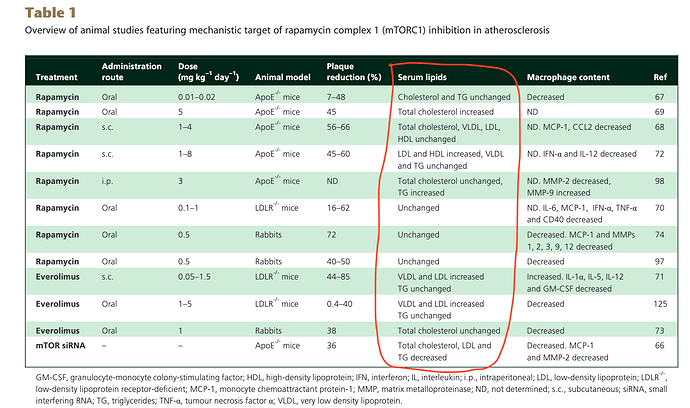
Potential therapeutic effects of mTOR inhibition in atherosclerosis (2015)
Many good references, in vitro, in vivo, and clinical translation.
With respect to the mTOR positive indications from lab models, this table reproduced herein. Looking at the “serum lipids” column, you see most all of the models, lipids and TG are unchanged? Yet, our current concern is clinical translation where we know in humans, higher dosing WILL elevate TG/lipids? So how does that change animal model translation?
“Unfortunately, rapalogs are known to trigger diverse undesirable effects owing to mTORC1 resistance or mTORC2 inhibition. These adverse effects include dyslipidaemia and insulin resistance, both known triggers of atherosclerosis. Several strategies, such as combination therapy with statins and metformin, have been suggested to oppose rapalog-mediated adverse effects” (combination therapy, more variables, interactions, risks)
Everolimus was apparently superior to Rapamycin, but most everyone here is on Rapamycin?
Side commentary on cancer in this paper, drilled down.
“When it was given chronically, however, rapamycin paradoxically led to glucose intolerance in mice, rats and humans. This effect was, at least partly, mediated by mTORC2 inhibition following chronic rapalog administration as mTORC2 has recently been identified to be a critical mediator for insulin sensitivity: another explanation is mTORC1 resistance following chronic rapalog treatment. In vitro experiments using renal carcinoma cells (RCC) revealed that long-term everolimus treatment results in hyperphosphorylation of S6rp, a crucial mediator in insulin resistance. ***Along this line, we have obtained in vivo evidence that chronic inhibition of mTORC1 in mice treated with everolimus paradoxically results in over-activation of mTORC1 as well as in diminished autophagy (Kurdi et al., unpublished results). The ability of rapalogs to shift their actions based on the duration of their administration or dosage is still poorly investigated, despite its importance in the clinic. In antitumour therapy, for example, chronic administration of rapalogs is known to induce resistance to the drugs through different mechanisms. So still many questions in human usage.”
I looked for the published results
Continuous administration of the mTORC1 inhibitor everolimus induces tolerance and decreases autophagy in mice
Everolimus at 1.5 mg·kg per day, various time models (about 9 mg/day for 70kg human)
KEY RESULTS
As expected, everolimus inhibited mTORC1 and stimulated autophagy in the liver after 3 days of treatment. However, continuous administration for 28 days resulted in hyperactivation of the Akt1-mTORC1 pathway accompanied by a remarkable decrease in autophagy markers. Everolimus given intermittently for 56 days partially rescued mTORC1 sensitivity to the drug but without inducing autophagy. The failure to induce autophagy following long-term everolimus administration was due to uncoupling of the mTORC1 substrate unc-51 like autophagy activating kinase 1." Yikes
CONCLUSIONS AND IMPLICATIONS
Our data encourage the use of intermittent everolimus regimens to prevent tolerance and to extend its activity
(my sense of the literature on cancer/transplant studies with Rapamycin, where results were not that great (another drill), patients would be considered to be on “chronic” administration, min trough/AUC level? But the seminal cancer/GFJ study measured mTOR inhibition early on, but did they follow these patients over a long period of time and re-measure mTOR??)
“A plausible alternative explanation (mTOR1 resistance) might relate to the resistance to everolimus following long-term treatment, which is a clinically relevant and well-described phenomenon in renal cell carcinoma (RCC), prostate cancer and some other tumour types. These tumours, while initially reacting well to everolimus therapy, often progress to a resistant form in the long run. It is therefore tempting to speculate that analogous to a tumour exposed to long-term mTORC1 inhibition, the high synthesis rate of liver cells drives the initiation of adaptation mechanisms, resulting in hyperactivation of the Akt1-mTORC1 pathway and drug tolerance”
Of course, they are high dose, chronic administration models. But how to reconcile this finding with CHRONIC “Rapamycin” mice studies (mTOR is inhibited), where cancer is delayed, and longevity increased? In humans, clearly chronic administration leads to resistance? It’s a matter of dosing and the “right” mTOR inhibition level…balancing cancer and CAD risks??
Without TOR assays, flying blind…
The paper RadAdmin linked to:
Gliflozins in the Management of Cardiovascular Disease
Lots of clinical trials referenced, all for diabetics, CKD, and/or persons with established CAD. Nothing of course on healthy persons.
I don’t have diabetes, CAD, and my last eGFR was 100. Plus I’m ketogenic, already very low glucose, and 24 hr intermittent fasting. Would I take this drug (cost aside)? Would I go hypoglycemic…probably.
“SGLT2 inhibition raises circulating ketone levels, an effect that appears to improve mitochondrial function, increase the production of ATP, and enhance ventricular contractile performance”
“Diabetic ketoacidosis, which is relatively uncommon, may occur, particularly in elderly patients with volume depletion. The disorder may be precipitated by an acute illness or fasting and may be accompanied by serious hypotension. Diabetic ketoacidosis is characterized by an accumulation of ketone bodies, principally hydroxybutyric acid. A form of ketoacidosis in patients receiving SGLT2 inhibitors is euglycemic ketoacidosis, which is not accompanied by a markedly elevated blood glucose levels”
@RapAdmin Seems you’re the SGLT2 expert, aside from ITP, is much of the mice research on healthy wild mice, or some dsyfunctional diabetic models? Also, do you know if mTOR is at all implicated or just glucose reduction, or other unknown?
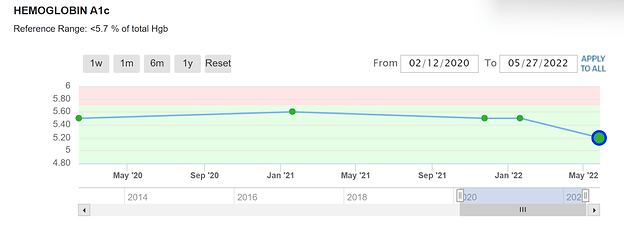
I have not noticed any increase in insulin resistance. At 81 my fasting glucose is a little higher than someone younger. In fact, my A1c appears to be improving. So far rapamycin has appeared to be benign when it comes to insulin resistance.
My diet is nothing special, somewhat South Beach, but I do 18/6 time-restricted eating and take Metformin 500 mg twice daily. Since the last test, I have added Jardience 10 mg before each meal. I have ordered Jardience 20 mg which I will be taking from now until I see the results of my next tests.
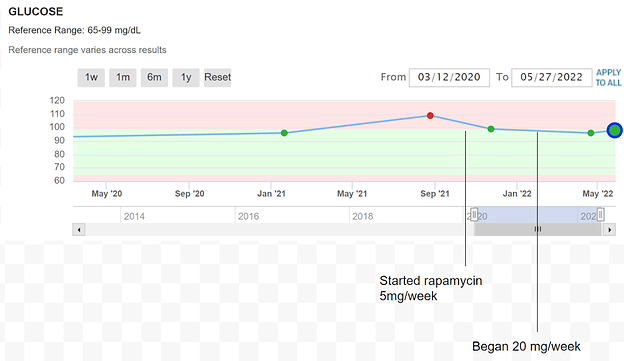
My last fasting glucose and Hemo A1c were taken after ~4 weekly doses of 20 mg rapamycin with grapefruit juice. I have cut back to 12mg with grapefruit juice because the 20 mg dose was giving me mild diarrhea.
Well not quite “Rapamycin is benign” since you’re doing a mixed Rapamycin/1000mg Metformin. But indeed, no signs of glucose dyregulation, just some elevated lipids (past posting). What would your glucose and lipids show without metformin/statin?
Weekly or every 2 weeks?
Jardience add on, will be very interesting.
Thanks for sharing your n=1 journey results, very informative for the community.
100%. But even with a low risk profile, I’d still get a CAC to establish definitively one’s true status if prevention/longevity is the game plan.
The major cause of death and almost NO ONE knows their true underlying heart status/risk?
Dosing weekly at this point. I started taking metformin when my fasting glucose and A1c were in the normal range. The same with atorvastatin, I started taking it when my lipids were in the “normal” range.
[quote=“MAC, post:378, topic:1434”]
CAC
[/quote]I agree that the CAC scores are important. What I cannot understand is why my cardiologists, primary care doctor, and insurance company don’t seem to recognize this or care. As far as I can tell, most if not all insurance companies won’t pay for the test. Why is this?
The coronary calcium score contributes to stratifying the risk of complications in COVID-19 patients. Cardiovascular calcifications appear to be a promising imaging marker for providing pathophysiological insight into cardiovascular risk factors and COVID-19 outcomes.
https://www.nature.com/articles/s41440-021-00798-9
strong text
This is very interesting and tolerance to mTOR inhibition is worth considering. Why are we dosing weekly or even biweekly? Is there data to support that?
There may actually be data to question it.
Here’s the Matt study showing great results in mice giving a brief duration of drug and lasting a long time
And here’s a recent look at Drosophila showing a long memory of pulsed mTOR inhibition on the gut
I know when I first started rapamycin 5 years ago I discussed it with Alan Green for several hours . He basically guessed at the dose and dosing schedule. It was gutsy and worked out, but it was a guess.
I also feel that it may be weaker now but rather than keep upping the dose we should be pulsing it to prevent tolerance.
The studies you referenced MAC should be considered.
Maybe we should be taking a fairly hefty dose every 3-6 months.
People will argue that tolerance is only in chronic users, but aren’t we chronic when we take a drug with a long half life regularly over many years? Perhaps pulsing it less frequently would eliminate the glucose, lipid, and other concerns that may have.
It’s probably worth a post of its own.
Because we dose for longevity using PFA/WAG/SWAG!