Interesting: Rajagopal V. Sekhar, the author of the study that found a 24% lifespan increase in mice (see here for analysis and caveats: Glycine+NAC vs Rapamycin ), published a subsequent paper last year: GlyNAC (Glycine and N-Acetylcysteine) Supplementation in Old Mice Improves Brain Glutathione Deficiency, Oxidative Stress, Glucose Uptake, Mitochondrial Dysfunction, Genomic Damage, Inflammation and Neurotrophic Factors to Reverse Age-Associated Cognitive Decline: Implications for Improving Brain Health in Aging
The PTEN-induced kinase 1 (PINK1) is a mitochondrial-targeted kinase that recruits the E3 ubiquitin ligase Parkin to mitochondria to initiate mitophagy. This study found decreased protein expression of PINK1 suggesting decreased mitophagy in the aging brain. GlyNAC supplementation led to the recovery of PINK1 suggesting improvement in brain mitophagy. Improving mitophagy in the brain would result in optimizing and improving overall brain mitochondrial function, and thereby improve brain health and cognitive function.
GDNF is identified as being important for preserving the health of dopaminergic neurons, with implications for Parkinson’s disease, but there appears to be limited clinical success of exogenously administered GDNF. Another interesting study reported that ischemia-injured neurons were rescued by astrocytic GDNF modulation [112] suggesting a wider role for GDNF in preserving and promoting brain health and recovery. Therefore, it is interesting to find that the aged mice in this study had low expression of GDNF in the brain and that GDNF expression improved to levels seen in young mice after GlyNAC supplementation. This finding indicates restoration of endogenous GDNF in their native environment rather than provision via exogenous administration. While it may be premature to consider the implications of GlyNAC supplementation in Parkinson’s disease based on this study which did not measure GDNF specifically in dopaminergic neurons, the findings of this study nevertheless support the need for future research on the effect of GlyNAC supplementation in dopaminergic neurons and signaling pathways.
The same team tried high-dose GlyNAC on older adults and found improvements in some aging hallmarks that are also Parkinson’s hallmarks: mitochondrial dysfunction, insulin-resistance, cognition, strength, and gait-speed:
-
Supplementing Glycine and N-Acetylcysteine (GlyNAC) in Older Adults Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Physical Function, and Aging Hallmarks: A Randomized Clinical Trial 2022
-
Glycine and N-acetylcysteine (GlyNAC) supplementation in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, insulin resistance, endothelial dysfunction, genotoxicity, muscle strength, and cognition: Results of a pilot clinical trial 2021
They’re also running larger trials of GlyNAC for AD, MCI, and Covid-19 recovery. Wait and see…
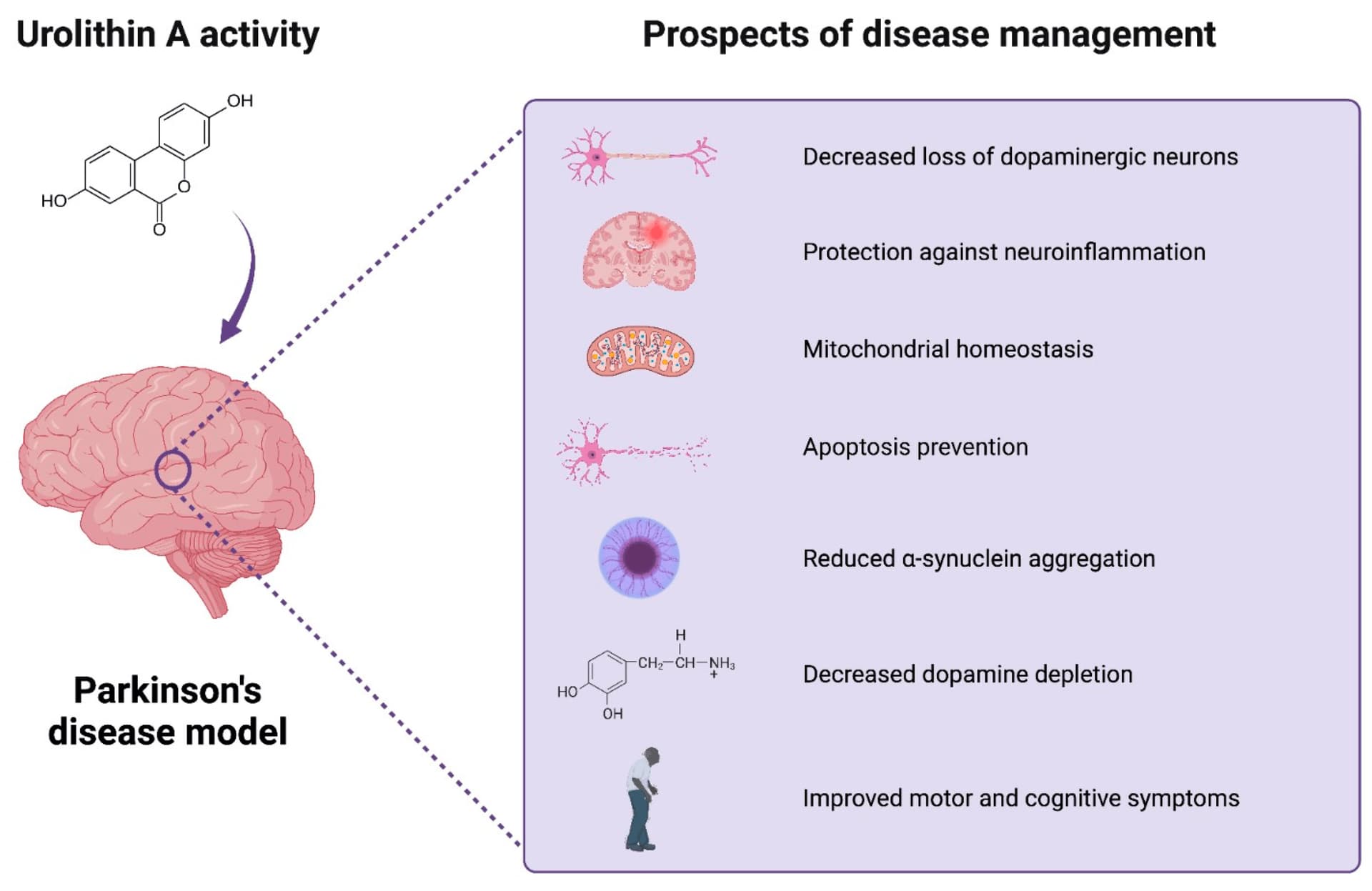
So I wonder if there could be benefits of GlyNAC in PD. This Nov 2023 paper notes: Mitochondrial dysfunction in Parkinson’s disease – a key disease hallmark with therapeutic potential
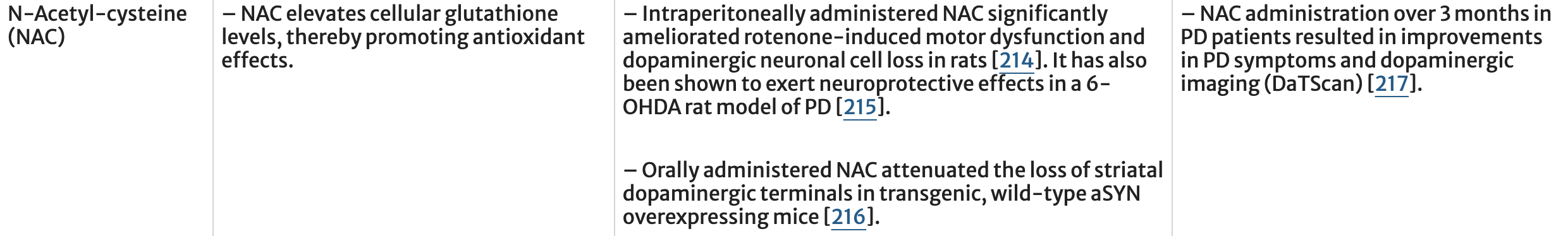
A related approach is to try and boost brain concentrations of glutathione (Table 2). Nigral levels of glutathione are lower in PD patients, possibly because of an increased reliance upon glycolysis for ATP production in PD patients. Elevating glutathione has been proposed and explored in preclinical and clinical trials. However, it is unclear whether this is simply an effect of mitochondrial dysfunction and whether adequate brain concentrations can be achieved with oral dosing. N-Acetyl cysteine (NAC), an approved drug to treat acetaminophen induced liver failure, increases cellular glutathione levels in vivo. Notably, weekly intravenous administration of NAC over 3 months in idiopathic PD patients revealed a significant clinical improvement which was paralleled by increased dopamine transporter binding during ioflupane imaging (DaTSCAN).
Oral glutathione ranked first in the 2023 Mischley survey (if you only include compounds with n > 50). But it seems better to give the body the building blocks of glutathione (glycine and NAC) than glutathione itself.
However, here they conclude: How to Increase Cellular Glutathione 2023
In addition, as a general concept, it also needs to be clarified whether there is sufficient evidence that increasing GSH levels is associated with improved prognosis or protection against disease. Increasing GSH levels is certainly possible, but there is little clinical evidence to support the impressive promises that theory and experimental research have made. In other words, it is not yet fully established whether increasing GSH levels is beneficial, as there are still no solid clinical trials to support this, and there are doubts about the efficacy of treatments in humans. It is not the aim of this review to investigate the reasons for the failures or uncertainties in this area, but we have limited ourselves to assessing which molecules might actually be able to increase GSH levels and by what mechanism, as some are likely to be much more efficient than others.
Would be great for the ITP to test GlyNAC…