And for AD:
And, related to Alzheimers…
UCSF leads first large-scale study to identify the characteristics of underdiagnosed syndrome.
A team of international researchers, led by UC San Francisco, has completed the first large-scale study of posterior cortical atrophy, a baffling constellation of visuospatial symptoms that present as the first signs of Alzheimer’s disease. These symptoms occur in up to 10% of cases of Alzheimer’s disease.
The study includes data from more than 1,000 patients at 36 sites in 16 countries. It published in the Lancet Neurology on Jan. 22, 2024.
PCSK9 decrease increases Alzheimer’s risk, mendelian randomization:
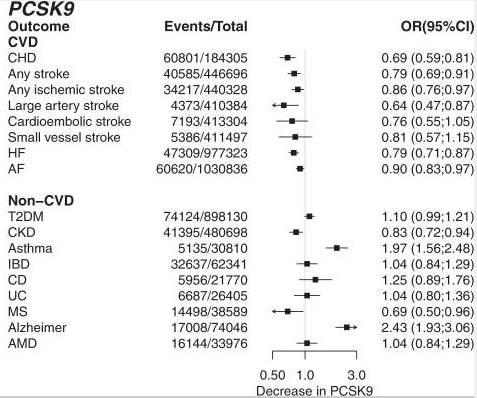
Not looking good for PCSK9 inhibitors for Alzheimer’s?
Thanks for calling attention to this. It is def something to look into. Did you see any other MR analyses consistent with?
(the authors of the paper may be rooting for CEPT that is the main focus of the paper so would be good to see other who may be less biased report on it).
Note that I saw this after a quick search…
By using an established BBB model, we identified reduced LRP1-mediated brain-to-blood Aβ clearance due to PCSK9 across different endothelial monolayer in vitro. Consequently, the repetitive application of FDA-approved monoclonal anti-PCSK9 antibodies into 5xFAD mice decreased the cerebral Aβ burden across variants and aggregation state, which was not reproducible in brain endothelial-specific LRP1−/− 5xFAD mice. The peripheral PCSK9 inhibition reduced Aβ pathology in prefrontal cortex and hippocampus–brain areas critically involved in memory processing—and prevented disease-related impairment in hippocampus-dependent memory formation. Our data suggest that peripheral inhibition of PCSK9 by already available therapeutic antibodies may be a novel and easily applicable potential AD treatment.
In accordance with detectable reduced Aβ pathologies in prefrontal cortex (Fig. 5A + C) and hippocampus (Fig. 5B)—brain structures, which are essentially participating in memory formation and retrieval [61, 62] -, targeting peripheral PCSK9 in 5xFAD mice preserves hippocampus-dependent learning capacities [39] to a level of control-treated wildtype littermates (Fig. 6A).
—
@A_User or others. Do you know if below might mean that taking a PCSK9i medicin means that we are not impacting PCSK9 level is the brain, whereas a Mendelian Randomization study does pick up the differences with people who expressed more PCSK9 in the brain - hence this is one of the cases where PCSK9 MR might not represent PCSK9 inhibitor antibody medicines.
Hence it could be that lower PSCK9 in the blood is neutral (or according to this paper perhaps good) for AD risk as it does not impact CNS levels, while someone who genetically has lower PCSK9 also would have lower in the CNS which might be what is negative as picked up by the MR paper that @A_User shared?
Because it has been shown that blood-circulating PCSK9 is not capable of crossing an intact BBB [63], we postulate that peripheral PCSK9 inhibition modulates cerebral Aβ burden via brain endothelial and peripheral LRP1 levels. Even though our data display a substantial impact of PCSK9 on endothelial LRP1 activity, we cannot attribute the significant reduction of cerebral Aβ and cognitive improvement in vivo solely to brain endothelial LRP1. The dramatic impact of LRP1BE−/− on Aβ pathology (Fig. 4A–D) and hippocampus-dependent learning behavior (Fig. 6A), might mask further potential beneficial effects of peripheral PCSK9 inhibition.
Ok, I read the paper you shared @A_User a bit more and they do seems to point to what I was asking above as a big question mark:
Findings such as the observed increased risk of AMD (from lower CETP), or of asthma and Alzheimer’s disease (from lower PCSK9), or the apparent protective effect on MS (from lower PCSK9) provide inference on the likely consequences of protein inhibition. However, whether pharmaceutical compounds targeting these proteins elicit similar effects depends on both the duration of drug exposure, as well as the potential for a drug to access the relevant tissues. For example, monoclonal antibody PCSK9 inhibitors may not cross the blood brain barrier.
(Where from the paper I shared it seems like it is more a “do not” generally cross the barrier vs “may not”)
So as of now I think the AD risks is more questionable than it first came across
Including that that is how the authors of the paper frame it themselves.
Still def something to look into more. Has anyone seen other evidence going either way?
—
Btw - in the paper you shared they say
These analyses suggest the risk-increasing effect of CETP on AMD was likely due to its HDL-C increasing effect, while the Alzheimer effect of PCSK9 was likely mediated by LDL-C (Fig. 6)
Do we know what they mean by this?
If is that LDL was lowered in brain => risk of AD, that may be what some have argued statins might cause (even if they might offset the risk more in other ways), while again PCSK9i medicines of today are not lowering LDL in the brain, but just in the liver/blood…
Only short term, but large and positive it seems:
Repatha® (Evolocumab) Phase 3 Cognitive Function Study Results Published In The New England Journal Of Medicine Study Showed Lowering LDL-C With Repatha Did Not Impair Cognition. Results From One of the Largest Cognitive Function Trials Support Safety Profile of Repatha
The study demonstrated that Repatha was non-inferior to placebo, with no significant difference in cognitive function between the Repatha and placebo-treated groups. “In the first prospectively designed study of cognitive function with a PCSK9 inhibitor using validated instruments, we showed that there were no significant differences between patients taking evolocumab and those on placebo,” said Robert P. Giugliano, M.D., S.M., Brigham and Women’s Hospital, Boston and lead study investigator. “These findings are reassuring for both physicians and patients because they show that LDL cholesterol levels can be lowered with evolocumab to levels well below current treatment targets, with no negative effects on memory or other cognitive domains.” The effect of Repatha on executive function (primary endpoint) was non-inferior to placebo, and there was no statistical difference between Repatha and placebo on the other cognitive domains tested: working memory, memory function and psychomotor speed (secondary endpoints). “Historically, the clinical cardiology community has had concerns that low LDL-C levels may impact cognitive function,” said Sean E. Harper, M.D., executive vice president of Research and Development at Amgen. “Across our comprehensive clinical trial program, thousands of patients have been treated with Repatha, which has demonstrated a consistent safety profile, even at very low LDL cholesterol levels. These findings add to the body of evidence supporting the safety of LDL-lowering with Repatha in patients with established cardiovascular disease who need more than statin therapy alone.” In the primary cohort of 1,204 patients, followed for a median of 19 months, the change from baseline raw score of spatial working memory strategy index of executive function was similar in the Repatha and placebo groups (mean baseline score 17.8; mean change from baseline -0.21 versus -0.29, respectively). [I have not read the paper but seems like the trend favors Repatha?]* The primary endpoint was below the pre-specified margin, demonstrating non-inferiority. The primary endpoint was assessed by the Cambridge Neuropsychological Test Automated Battery (CANTAB) Spatial Working Memory strategy index of executive function.
Secondary endpoint results in the three cognitive domains of working memory, memory function and psychomotor speed were consistent with the primary endpoint result, and patients treated with Repatha experienced changes from baseline similar to placebo in all three cognitive domains tested. Changes from baseline in the global composite score were also similar between treatment arms.
I will be looking into this in more detail very soon and reply, but I did look at this some while ago and reducing LDL in MR did show decrease in Alzheimer’s risk iirc. CETP inhibition also looked good.
I don’t trust papers from big pharma. Especially on AD. The way they measure cognitive function is debatable. We want to see long-term data on use and whether it extends lifespan. It’s fairly easy on patients >80yo and/or with AD as they have a high death rate so any significant positive impact would be identified if you have enough people.
I agree that we generally should be hesitatant about pharma papers (why it was the last of my three posts in a row). At the same time, there is a spectrum from when it eg is their own authors and done in a non-audited way vs this type of case when the paper was written only by academic professors / attending physicians at top academic institutions and represents dozens and dozens of academics co-investigators from many of the world’s top medical institutions and research hospitals who have reputations to balance (see pages 2-10 here https://www.nejm.org/doi/suppl/10.1056/NEJMoa1701131/suppl_file/nejmoa1701131_appendix.pdf)
Re ”We want to see long-term data on use and whether it extends lifespan”
Agree 100% on that. At the same time - we’d also want to long-term data showing that there is any AD risk right? There is nothing on that yet.
Re long-term relevant data on mortality / lifespan @A_User has shared
a lot of compelling data from Mendelian Randomization and other sources, suggesting that there is a causal all course mortality / longevity signal. (See massive cardiovascular thread). That MR data is more compelling than I think we have seen for almost any other medicine - as far as I know there very little MR at all on all cause mortality (vs distinct disease benefits) for most of the drugs discussed on this forum outside of cardiovascular meds. (The main one I know of is the SGLT1i MR study we have discussed).
(Perhaps at some point we on this forum - or some academic paper could summarize what drug pathways have MR data supporting not just disease but decreases in all cause mortality/ longevity).