Sorry for the 3rd post in a row but wow… I’ve just checked the supplemental material of the just published Association of cardiovascular disease management drugs with Lewy body dementia: a case–control study and table S5 shows that the 4th best-performing drug to prevent Lewy body dementia is… the immunosuppressant Tacrolimus! With a 1y OR of 0.46 [0.32-0.59]. Tacrolimus is not in their 3y model, but the 9th most performing in the 3y model is another immunosuppressant currently tested in the ITP: Methotrexate. This is surprisingly not mentioned in the paper itself.
I know, lithium causes tremors, but I think you’re right–more at the doses used to treat bipolar disorder, which is grams a day. The dose makes the poison, eh?
The problem is, my doctor thinks I’m nuts (you’re taking rapamycin!? That’s a dangerous cancer drug! You’re using exenatide!? It has side effects! ) so I have trouble getting him to listen to anything.
You know, having Parkinson’s has side effects. Growing old has side effects. We choose which ones WE want to risk.
Ooof. You ask hard questions.
Let’s see–I did try the whole NAC NMN thing, and I’m ashamed to say part of the reason I leapt on the Peter-Attia-It-Doesn’t-Cross-The-BBB-anyway conclusion was because I didn’t like the pills: they were big and smelled terrible.
Metformin. Also read along the way there wasn’t a lot of point. Also read some stuff about metformin vs. exercise…and I exercise like a crazy person.
Mannitol. There were some iffy, non-placebo controlled positive-ish results that I ultimately decided I didn’t buy.
Tryptophan–because I listened to a far-out theory by Dr. Jonathan Sackner-Bernstein and I’ll try anything. In a nutshell, he believes PD is lack of dopamine transport, not lack of dopamine. Since dopamine is toxic (thus killing their overstuffed neurons) he thinks the fix is to block synthesis. Ha. I felt awful pretty quickly.
Just FYI: Venglustat. I was in the Sanofi/Genzyme study, NOT on placebo, for nearly the full three years, before the study was stopped.
I take Azilect, which was all the neuroprotective rage when I was diagnosed. I know it’s kind of fallen out of favor. I’ve debated switching to seligiline.
Here’s what I think in general though: any treatment for the cause of PD (as opposed to the symptoms) isn’t gonna make you feel miraculously better. It’s going to keep neurons from dying, or maybe nurture baby ones, and I’m not convinced that’ll be detectable on a day-to-day basis.
If something makes you feel different overnight, to me it screams placebo.
I am musing on what I wouldn’t give up. I didn’t even MENTION my keto diet, or hot tub/cold pool, or exercise, or non-negotiable sleep routine. You see?
BTW I’ve had PD for 10 years.
Thanks for your detailed answer! What about non-motor symptoms like sleep and depression? (besides diet, hot/cold tub, exercise and sleep hygiene) (for the context: I’m interested in PD because I have a high polygenic PD & DLB risk…)
I agree. I’m getting more and more optimistic though. For instance, when I see the cognitive and gait improvement in this study with empagliflozin: Canagliflozin - Another Top Anti-aging Drug - #363 by adssx
I asked Cure Parkinson’s UK whether they were considering empagliflozin and they’ve just answered:
Empagliflozin came in 3rd place at last summer’s iLCT meeting. The committee are very keen to see it progress to clinical trial in PD and suggested that a small Phase 1b biomarker study exploring target engagement and confirming brain penetrance in a PD cohort would be useful. But there were concerns around what target engagement looks like in PD. So there were suggestions that more preclinical work required on potential mechanisms of action. Empagliflozin has only been preclinically tested in neurotoxin models of PD, so the committee felt that there was a need for testing in a synuclein model. In terms of costings, the preclinical work is probably £200K, while Phase 1b would be ~£500K.
If anyone wants to contribute, here’s how to donate (there are tax-efficient ways to donate for US and UK taxpayers) ![]() (feel free to DM me as well, I can put you in touch with the Cure PD team)
(feel free to DM me as well, I can put you in touch with the Cure PD team)
Well, I think you might have snuck into my house and gone through my cabinets.
Yes, I take Simvastatin. My cholesterol levels were pretty high, probably because I eat keto, and I have been eating keto since my diagnosis. (The statin works though–my levels are good now.) And, because of the statin I also take coQ10.
And thinking on it, you asked about the one thing I would not give up and I would probably say that: my diet. Also, before rapamycin I used to do four-day fasts. I still practice time-restricted eating, sometimes OMAD. And now I take Acarbose, which allows me to cheat a little…berries and such. I wear a CGM, not all the time, but enough to have a sense of what I can eat.
For whatever reason, I have been VERY lucky: I sleep well, and I am not particularly depressed. My right arm is stiff, and when I am nervous, my right hand shakes. Of course I get instantly nervous whenever I want to show someone how unaffected I am.
Interesting that you have a “pure” motor PD subtype. I wonder whether it’s different to treat.
If you’re on a keto diet and often fast then SGLT2 might be counter indicated because of the DKA risk.
I don’t understand how ambroxol may work in PD. It looks like ambroxol inhibits autophagy ![]() Effects of ambroxol on the autophagy-lysosome pathway and mitochondria in primary cortical neurons 2018:
Effects of ambroxol on the autophagy-lysosome pathway and mitochondria in primary cortical neurons 2018:
This was not expected as ambroxol has been shown to increase macroautophagy in human neuronal cells containing GBA1 mutations. The lower dose or different type of neuron might account for the discrepancy. […] The mechanism by which TFEB is activated following ambroxol treatment in mouse cortical neurons requires further investigation. For example, is it a compensatory mechanism due to the inhibition of macroautophagy following ambroxol treatment?
This 2023 study also showed a HIGHER risk of Parkinson’s following ambroxol use at the normal dose of 90 mg/day (vs. about 10x more in the PD trials): Association Between Ambroxol at the Usual Dose and the Risk of Parkinson’s Disease in Chronic Lung Disease
Any idea @momgotshocked?
High-dose NR looks interesting, higher doses may be needed if it does not cross the BBB well?
- Vitamin B3 supplement shows early promise for Parkinson’s 2022: “1000mg of nicotinamide riboside daily for 30 days […] The supplement also showed promising signs that it may improve metabolism and reduce inflammation in the brain, which could have protective effects in the brain. And the participants who showed the greatest increase in NAD levels also showed some mild improvements in their Parkinson’s symptoms.”
- NR-SAFE: a randomized, double-blind safety trial of high dose nicotinamide riboside in Parkinson’s disease 2023: “NR 1500 mg twice daily […] The trial met all prespecified outcomes. NR therapy was well tolerated with no moderate or severe adverse events, and no significant difference in mild adverse events. NR therapy was associated with clinical improvement of total MDS-UPDRS scores. However, this change was also associated with a shorter interval since the last levodopa dose. NR greatly augmented the blood NAD metabolome with up to 5-fold increase in blood NAD+ levels.”
It also ranked near the top in Mischley’s survey: Parkinson Symptom Severity and Use of Nutraceuticals (“NAD+ or its precursors”)
There are two ongoing trials, ending in December 2024, so results expected next year:
Nicotinamide riboside failed in the ITP in 2016 (1000 ppm, started at 8 mo, 1000 ppm for mice means about 1,400 mg for a 60 kg human if I’m not wrong). However, interestingly, the ITP still decided to test NR again in 2022, this time in a higher dose of 2400 ppm (so about 3,333 mg for a 60 kg human) and combined with metformin. I don’t know what their reasoning was, but there might be something here for PD in glycemic control (with low-dose metformin, GLP1RA, or SGLT2, probably to protect the neurons?) + NR (to promote neurogenesis?).
@TomParkinson: I think you’ve been testing high-dose NR for a few weeks, how is it going?
I wonder what else could promote neurogenesis, some ideas:
- Creatine: it failed to show improvement in motor symptoms of PD (The effectiveness of creatine treatment for Parkinson’s disease: an updated meta-analysis of randomized controlled trials 2017), but it may work for non-motor symptoms and especially depression (Creatine, a popular exercise supplement, might help treat depression).
- Astaxanthin: worked in the ITP + Astaxanthin supplementation enhances adult hippocampal neurogenesis and spatial memory in mice + Astaxanthin ameliorates dopaminergic neuron damage in paraquat-induced SH-SY5Y cells and mouse models of Parkinson’s disease 2023
- Taurine: not yet tested in the ITP (but according to other authors: “The median life span of taurine-treated mice increased by 10 to 12%”, with the equivalent of 3g/d for humans) + Taurine increases hippocampal neurogenesis in aging mice
The initial theory about ambroxol (as far as I know) was that it only acted as a chaperone for missing/improperly-folded glucocerebrosidase. Glucocerebrosidase helps to digest particular sugars in the lysosomal membrane when it inverts and forms little vesicles of cellular garbage for digestion. If the vesicles aren’t digested, the lysosomes eventually fill up with their own membrane bits–no room left for anything else. It sure sounds like ambroxol would improve macroautophagy, doesn’t it?
And there seemed to be some evidence that many people with PD have low G-Case levels, whether or not they have a GBA mutation. I’m not sure I would bother if I didn’t, though.
BUT, just recently I read that ambroxol does something else, at the mitochondrial level. I think Simon Stott wrote an article on it in Science of Parkinson’s. I need to dig that paper up. And yes, if it’s a dose thing, I take over a gram a day.
I wish I knew…
Indeed, thanks for mentioning it, I hadn’t read it: Just a lysosomal enzyme… – The Science of Parkinson's
New seminal paper reporting immunological shifts during early-stage PD: Immunological shifts during early-stage Parkinson’s disease identified with DNA methylation data on longitudinally collected blood samples 2024
Additionally, we noted previously unrecognized decreases in the naive B cell compartment in the defined PD and Prod patient group. Over time, we observed the proportion of innate immune cells in PD blood increased, but the proportion of adaptive immune cells decreased. We identified decreases in T and B cell subsets associated with REM sleep disturbances and early cognitive decline. […] Neutrophils can increase the permeability of the blood-brain barrier (BBB), giving all immune cells increased access to the CNS […] Comparing PD to HC, we found an increase in neutrophils and a decrease in monocytes and eosinophils. In PD patients, there is an increase in neutrophils and monocytes over time, with a consistent trend in the Prod group as well. We observed a slight decrease in monocytes in more symptomatic PD patients. […] We identified a strong decrease in CD4+ memory T cells and naive B cells in clinically defined and prodromal PD. In PD patients, we see a significant decrease in many adaptive immune cell populations over time, including all naive lymphocytes and memory T cells. In the prodromal group, there is a significant decrease in the naïve lymphocytes but not a significant trend in the memory compartments.
How can rapamycin impact the above? I checked a bit on the forum and most users report no changes in blood measures of neutrophils, lymphocytes, monocytes, eosinophils & basophils. (e.g., Concerns about immune suppression - #9 by RapAdmin ).
However, the most important finding in this paper seems to be the “strong decrease in CD4+ memory T cells and naive B cells” and these are hard to find according to @RapAdmin: Using HBA1C and LDL to Determine Ideal Rapamycin Dosage - #84 by RapAdmin
Another study found a similar result: Association Between Use of Any of the Drugs Prescribed in Norway and the Subsequent Risk of Parkinson Disease 2023
Of the 10 classes related to a lower risk of PD, there were 2 classes associated with a reduced PD risk of 30% or more, which were other nervous system drugs (N07) and antineoplastic drugs (L01). […] Similarly, the lower previous use of antineoplastic drugs (L01) among PD cases might also be related to a lower risk of smoking-related cancers in this group.
The L01 category features the usual suspects: ATC code L01 - Wikipedia
- L01BA Folic acid analogues such as methotrexate (currently tested in the ITP)
- L01EA BCR-ABL tyrosine kinase inhibitors such as dasatinib, nilotinib, and bosutinib
- L01EG Mammalian target of rapamycin (mTOR) kinase inhibitors (temsirolimus, everolimus, ridaforolimus, and sirolimus)
I’ve recently looked in the forum for data re: neutrophils because mine are elevated (not the absolute count but the %). In my case that’s because of a near chronic bladder infection I’ve been dealing with and only recently identified as such (the symptoms were UTI after UTI after UTI which basically means it never went away). In any case I looked specifically for that metric as I’m thinking more in terms of forensic clues for myself.
And rapa users are reporting DECREASED neutrophils while on rapa. This is anecdotal but I did hunt out pretty extensively on the forum and it seemed to be clearly a trend.
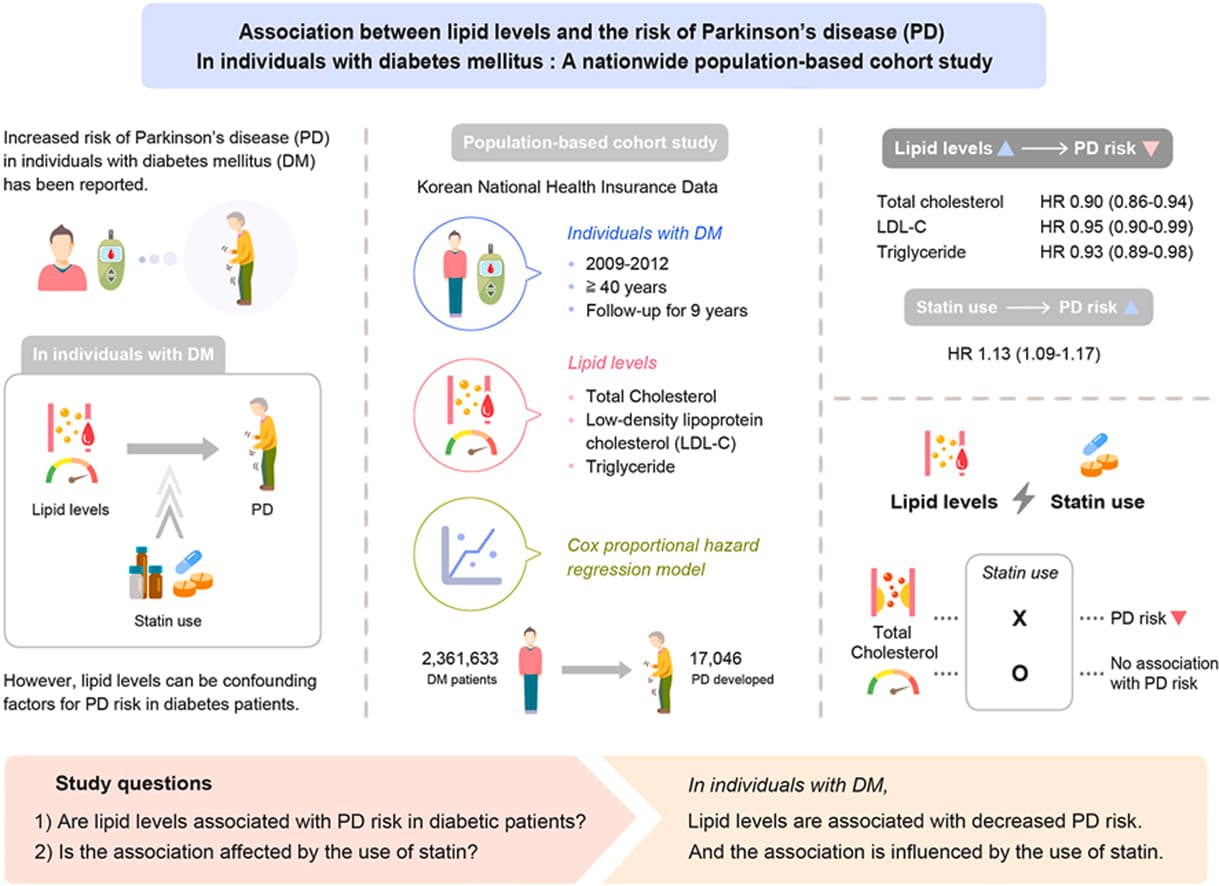
Continuation of lipophilic statins like atorvastatin is associated with decreased risk of Parkinson’s:
Results: Among the 43,810 statin initiators, the incidence rate for PD was 1.68 and 3.52 per 1,000,000 person-days for lipophilic and hydrophilic statins, respectively. Continuation of lipophilic statins was associated with a decreased risk of PD (hazard ratio [HR] 0.42 [95% confidence interval 0.27–0.64]) as compared with statin discontinuation, which was not modified by comorbidities or medications. There was no association between hydrophilic statins and occurrence of PD. Among lipophilic statins, a significant association was observed for simvastatin (HR 0.23 [0.07–0.73]) and atorvastatin (HR 0.33 [0.17–0.65]), especially in female users (HR 0.11 [0.02–0.80] for simvastatin; HR 0.24 [0.09–0.64] for atorvastatin). As for atorvastatin users, the beneficial effect was seen in the elderly subgroup (HR 0.42 [0.21–0.87]). However, long-term use of statins, either lipophilic or hydrophilic, was not significantly associated with PD in a dose/duration-response relation.
lee2013.pdf (268.2 KB)
Our study provides evidence that statins, especially atorvastatin, can reduce the risk of PD.
Possible prevention but not treatment?
This study suggests that statin use may have a detrimental effect on baseline nigrostriatal dopamine degeneration and long-term outcomes in patients with Parkinson’s disease.
Yeah, probably doesn’t work for treatment…
Findings In this randomized clinical trial, a double-blind, parallel-group, placebo-controlled futility trial involving 235 participants from 23 sites within the UK, participants in the simvastatin group had an additional deterioration in Movement Disorder Society Unified Parkinson Disease Rating Scale part III scores while not taking medication at 24 months compared with those in the placebo group (−1.52 points).
Some of the articles you shared are old. As you wrote, statins don’t work as a treatment; they cannot slow down the progression of Parkinson’s even in super early cases of PD who are not yet on med. It actually seems that high LDL is protective against PD (like obesity, diabetes, smoking and drinking coffee…) and that some statins might even be detrimental:
Effects of statins on dopamine loss and prognosis in Parkinson’s disease 2021
This study suggests that statin use may have a detrimental effect on baseline nigrostriatal dopamine degeneration and long-term outcomes in patients with Parkinson’s disease.
Parkinson’s Disease Progression and Statins: Hydrophobicity Matters 2022
This study suggests that hydrophilic, but not lipophilic, statins may be associated with faster PD progression.
(hydrophilic statins include fluvastatin, rosuvastatin, and pravastatin)
The use of statins was lower in patients who developed PD and higher in patients who developed DLB compared to patients with AD. In patients with PD, the use of statins was associated with the development of dementia in the 5 years following PD diagnosis.
Friends of mine who are Parkinson’s researchers wonder whether the protective effect of statins seen in some older studies might just be linked to an increase in glucose levels and some confounders like diabetes and obesity: Statins use => (associated to or causing) More diabetes => Protection against PD. That’s a pure assumption; they’re crunching massive datasets at the moment to confirm or infirm the hypothesis.
Statins direct Acetyl-CoA away from creating cholesterol. This provides more Acetyl-CoA for other purposes. Other purposes include acetylation of the histone.
An alternative to inhibiting the creation of cholesterol is to increase the amount of Acetyl-CoA.
Atorvastatin, one of the lipophilic statins, did have a good association, and it has a lower incidence of diabetes compared to rosuvastatin, which is hydrophilic, about half the rate.
A good association wasn’t dependent on LDL lowering but statin use, so it might be pleiotropy driving the association.
If nicotine fails as treatment, it’s not surprising to me that statins do as well. If you believe nicotine works as prevention.
Smoking prevents PD, not nicotine. Here’s a comprehensive review published 3 days ago in Movement Disorders: Clearing the Smoke: What Protects Smokers from Parkinson’s Disease?
The therapeutic potential of non-nicotine components of smoke is suggested by studies supporting multiple alternative mechanisms ranging from monoamine oxidase inhibitors to gut microbiome disruption to antioxidant response induction by chronic exposure to low levels of carbon monoxide.
More than 200 years since the publication of James Parkinson’s An Essay on the Shaking Palsy (1817), many uncertainties regarding the progressive neurological disorder now known by his name remain. This issue of The Lancet carries our first-ever Series dedicated to Parkinson’s disease, which includes an exploration of some of the outstanding questions around the epidemiology, causes, and current treatment options for this disabling and currently incurable condition.
Parkinson’s is second only to Alzheimer’s disease in the list of most common neurodegenerative disorders and, with increasing life expectancies and fewer competing causes of death, its prevalence is expected to increase
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(24)00094-1/fulltext
Thanks for sharing.
The cause of Parkinson’s disease is multifactorial and, although there is consensus among experts that Parkinson’s is an age-related disease, questions regarding the extent to which Parkinson’s can be attributed to external drivers (such as pollutants) do not yet have clear answers.
I agree, and I think that curing Parkinson’s might be the first “easy” step in the long quest to cure ageing…
(Among the authors of the series, there’s Tom Foltynie, who’s behind the trials of exenatide for Parkinson’s btw)
Good review of diet and supplements for PD: The Role of Diet in Parkinson’s Disease 2024
My tl;dr after skimming through it (let me know if I made a mistake):
- Mediterranean: good
- MIND diet: good
- Vegan: neutral, quality matters (“A recent UK biobank analysis found an association between a healthful plant-based diet and reduced PD risk (HR = 0.78 (95% CI: 0.61–0.99)), whereas an unhealthy plant-based diet was associated with a higher risk to develop PD (HR = 1.38 (95% CI: 1.08-1.74))”)
- Keto: potential benefits but challenging and safety unclear
- Dairy: bad? because of pesticides?
- Alcohol: good? (but there might be confounders)
- Coffee: good (but why?)
- Vit D: useless?
- Vitamin E and omega-3 fatty acids: good?
- Vitamins B6, B9, and B12: might be good?
- Vitamin B1:

- Vitamin C: enhances levodopa absorption
- Citicoline:

- Fiber, prebiotics, and probiotics: might be good? but huge heterogeneity in the bacterial strains, dosages, treatment durations, and methods of administration between included trials
- Mucuna pruriens: not recommended