@rapadmin Thanks for the reference.
@Joseph Thank you for the DSMO study and being an oral/IV DSMO guinea pig.
“Intranasal delivery means rapid, noninvasive access to the brain enabling numerous
novel therapies. However, this is not a panacea, and careful optimization will be needed
for any of these new treatments to reach patients. A major factor will be the formulations
and devices described in this article.”
ok expected that
Some “engineering” parameters to consider.
“Hydrophobic and charged hydrophilic molecules have been shown to diffuse poorly through mucus, whereas uncharged hydrophilic molecules are able to diffuse rapidly through the mesh of mucins nearly the speed of water for smaller molecules. Drugs larger than 500 Da in size will be especially prone to poor mucus diffusion and becoming stuck, though most drugs will be smaller than 500 Da in size, thus it is not an important issue. Optimizing a formulation to maximize crossing into the lamina propria will be crucial for any therapy. This size limit is not entirely inhibiting though, as molecules as large as wheat germ agglutinin–horseradish peroxidase (80 kDa) and even whole stem cells have been transported to some degree. Nonpolar compounds are thought to be transported poorly to the CNS intranasally, though there is a growly body of evidence that the proper microemulsion formulation can greatly increase the intranasal brain area under curve (AUC) compared to IV administration of the compound. Indeed, there is evidence that with some drugs increasing the hydrophobicity can increase delivery to the CNS. It is known that hydrophobic compounds cross biological membranes such as the nasal epithelia, blood vessels, or BBB well. This shows that not only are hydrophobic drugs capable of being administered intranasally with the correct formulation, but this may be an advantage. Often, researchers are administering doses as low as 25 µL but usually closer to 200 µL in size in these experiments; a size selected because this is the maximum volume of the nasal cavity in the model rodents. In humans, the nasal cavity is 6–7 mL in volume, which is impractical at best. Furthermore, 50% of the rodent nasal cavity is covered in olfactory epithelia, compared to <5% in humans. This limitation in area will make delivery to the CNS less efficient and adds emphasis on making sure administered drugs reach the correct region of the nasal cavity. Intranasal administration devices are another compelling strategy that will find a role in the clinical use of intranasal drugs. Recall that the olfactory region is <10% of the entire nasal cavity and located on the superior aspect as well as the rapid mucus clearance in the motile respiratory regions. Traditional spray pumps tend to only reach the anterior and lateral aspects of the nasal cavity, with <3% of the dose reaching the olfactory region.”
Rapamycin is both hydrophobic and a HUGE molecule…26,000 Da. But clearly in the study, no problem getting into the brain. Intranasal bypasses the entire gastric pathway, degradation loss, the massive system dose (and unwanted peripheral side effects) needed for therapeutic brain Rapamycin levels.
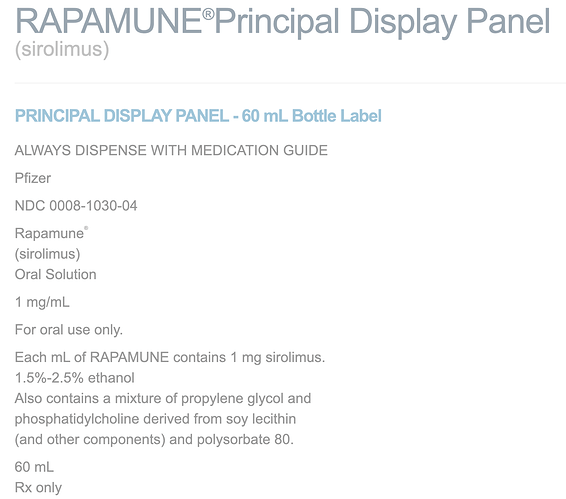
In the study, they used “InRapa” by Pfizer. I could not find “InRapa”, is this the same as their oral solution?
https://www.pfizermedicalinformation.com/en-us/rapamune/principal-display
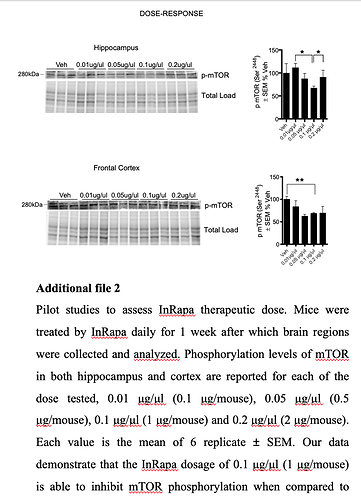
“The treatment was conducted 3 times per week, with a dose of 0.1 μg/μl of rapamycin solution or vehicle in 10 μl (1 μg/mouse) for 90 days total”. 6 mice were treated by single intranasal delivery of rapamycin, with the dose of 1 μg/mouse (0.05 mg/Kg/mouse)"
Based on the 1 ug/ul reference, this is the same as 1mg/ml oral Rapamycin solution, so I am assuming same.
That’s a super lower dose intransal to get large brain Rapamycin levels! Consider in some of the mice longevity studies, oral dose 2.25 mg/kg to 8 mg/kg. "To note, InRapa dose is about 50-times lower than i.p injection dose"
“Collectively, our data demonstrate that rapamycin delivered by intranasal route reached a therapeutic brain concentration comparable to that obtained by i.p. injection but with an extremely lower distribution at plasma level. Therefore, these results, coupled with the analysis by WB of mTOR inhibition in liver and heart tissue, which showed no changes between Ts65Dn rapamycin and vehicle treated groups”
“Overall, our data show that intranasal delivery of the mTOR inhibitor rapamycin was able to target and modulate mTOR kinase activity in the hippocampus. Our analysis of mTORC2 activity, indexed by phosphorylation of mTOR at Ser2481, show no alteration between Eu and Ts65Dn mice either with or without InRapa administration (supporting the low sensitivity of mTORC2 to rapamycin treatment”
Notice the 0.2 ug/uLdid not reduce pMTOR more than the 0.1 ug/uL.
Converting 0.05 mg/kg mouse to HED = 0.29mg @ 70 kg human (if oral or ip is equivalent to intranasal)
I think I won’t reinvent the wheel, make up a DSMO/water/Rapamycin powder solution and find a good intranasal device to deliver where it needs to go.
Now the black hole…qty, dose, and what the heck am I measuring as response?
Maybe will circle back and review some of those online tools…do some baselining, then re-test.
![]() Police might take a suspicious look…
Police might take a suspicious look…