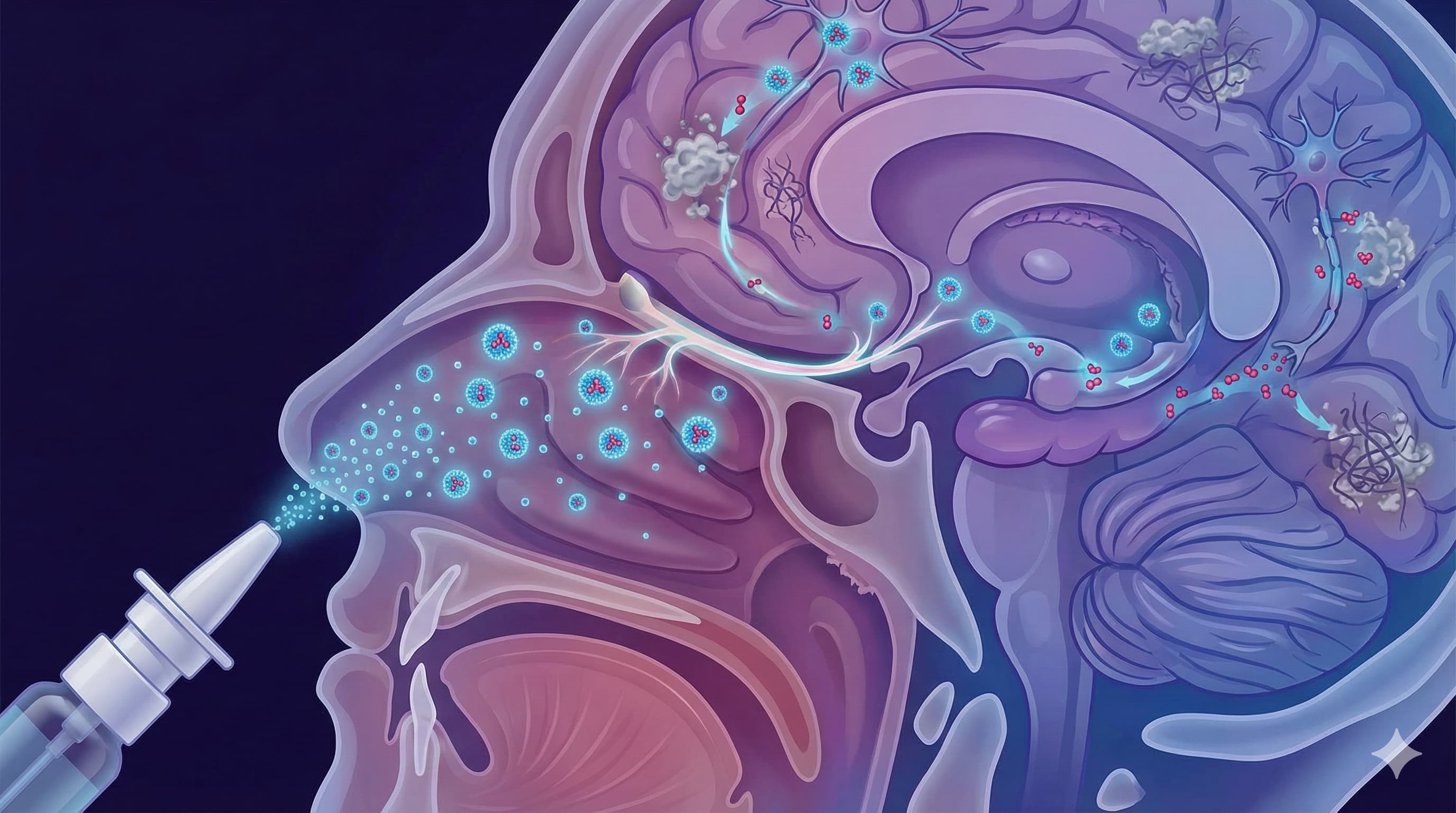
Researchers at Long Island University (USA) have published a compelling proof-of-concept study in the International Journal of Pharmaceutics demonstrating that intranasal delivery of rapamycin—encapsulated in specialized “brain-targeting” micelles—can reverse markers of Alzheimer’s disease (AD) in mice.
Rapamycin (sirolimus) is the gold-standard longevity drug, known for extending lifespan in every model organism tested by inhibiting the mTOR pathway. However, its translation to human neuroprotection is hampered by the Blood-Brain Barrier (BBB). To get enough rapamycin into the brain via oral tablets to clear toxic amyloid plaques, patients often require high doses that suppress the immune system or cause metabolic dysregulation (e.g., insulin resistance).
The “Big Idea” here is a bypass road. The team engineered polymer nanoparticles (micelles) tagged with a peptide (FibCS1) that acts like a VIP pass for the nasal lining and potentially the brain’s vasculature. When squirted into the noses of mice genetically engineered to develop aggressive Alzheimer’s (3xTg-AD), these nanoparticles didn’t just reach the brain—they reduced neuroinflammation (TNF-α, IL-6) and amyloid-beta (Aβ) plaques, and restored memory function. Crucially, they achieved this with a fraction of the typical oral dose, theoretically sidestepping the systemic toxicity that worries longevity enthusiasts.
While this offers a tantalizing “hack” for delivering geroprotectors directly to the command center, the technology relies on a proprietary delivery vehicle not yet available to humans.
Source:
- Paywalled Paper: Intranasal delivery of rapamycin via brain-targeting polymeric micelles for Alzheimer’s disease treatment
- Impact Evaluation: The impact score of the International Journal of Pharmaceutics is ~5.8 (Impact Factor) / 9.6 (CiteScore). Evaluated against a typical high-end range of 0–60+ for top general science, this is a High impact specialist journal (Q1 in Pharmacology), indicating rigorous peer review within drug delivery sciences
Part 2: The Biohacker Analysis
Study Design Specifications
- Type: In vivo (Murine) and In vitro (RPMI-2650 nasal epithelial cells).
-
Subjects: 3xTg-AD Mice (Triple Transgenic Alzheimer’s Model).
- Note on Sex/N-number: Specific sex breakdown for this trial was not detailed in the provided abstract, but 3xTg studies typically prioritize females due to more severe pathology.
- Dose Protocol: Intranasal administration of 0.2 mg/kg, every 4 days (q4d) for 5 total doses (20-day duration).
- Lifespan Data: N/A. This was a short-term acute efficacy study (20 days), not a longevity survival curve.
Mechanistic Deep Dive
- Primary Pathway: mTOR Inhibition. The study leverages rapamycin’s ability to induce autophagy (cellular cleanup). By dampening mTORC1 in the brain, the treatment upregulates the clearance of aggregated proteins (Aβ plaques) that suffocate neurons.
- Targeting Vector: The FibCS1 peptide targets fibronectin variants often upregulated in inflamed tissues and vascular endothelium. This suggests the micelles are not just passively drifting up the olfactory nerve, but actively latching onto mucosal/vascular entry points.
- Inflammaging Control: The treatment significantly lowered brain levels of TNF-α and IL-6. This confirms that local mTOR inhibition successfully quells the “cytokine storm” associated with neurodegeneration, a key priority for organ-specific aging.
Novelty
- The Vehicle: The use of FibCS1-PEG-b-PLA micelles is the true novelty. Generic intranasal rapamycin (without micelles) has been tried, but these nanoparticles showed superior nasal permeation (monomodal size ~98 nm) and stability.
- The “Micro-Dose” Efficacy: Achieving robust plaque clearance with just 5 doses of 0.2 mg/kg (spaced 4 days apart) challenges the assumption that chronic, daily dosing is required for neuroprotection.
Critical Limitations
- Aggressive Model Bias: 3xTg mice are an extreme model of AD. Humans rarely have three simultaneous mutations driving amyloidosis. Success here does not guarantee success in sporadic, age-related human Alzheimer’s.
- Short Duration: A 20-day study cannot assess long-term safety. We do not know if these micelles accumulate in the olfactory bulb or cause local necrosis over months of use.
- No Systemic Comparison: The study claims reduced systemic toxicity based on the logic of lower dosing, but detailed systemic toxicity data (e.g., glucose tolerance tests, immune cell counts) for this specific cohort versus an oral control group appears limited in the summary.
Part 3: Actionable Intelligence
The Translational Protocol
-
Human Equivalent Dose (HED):
- Animal Dose: 0.2 mg/kg (Mouse).
- Conversion Factor: Divide by 12.3 (standard FDA BSA conversion for Mouse to Human).
- Math: 0.2 mg/kg/12.3≈0.016 mg/kg.
- For 60kg Human: 0.016×60≈1.0 mg per dose.
- Frequency: Every 4 days.
- Biohacker Note: This is remarkably close to standard low-dose oral protocols (e.g., 2–6 mg/week), but applied intranasally, the brain concentration would theoretically be multiples higher than what 1 mg oral could achieve (due to ~1% oral brain bioavailability).
Pharmacokinetics (PK/PD)
- Bioavailability: Oral rapamycin has poor bioavailability (~14%) and is a substrate for P-glycoprotein efflux pumps at the BBB. Intranasal delivery via the olfactory/trigeminal nerves bypasses the BBB and first-pass hepatic metabolism.
- Half-Life: Rapamycin has a half-life of ~60 hours in humans. An “every 4 days” (96 hours) protocol allows for a “washout” trough, preventing continuous mTOR suppression (which is immunosuppressive).
Biomarker Verification Panel
To validate this protocol in a self-experiment (N=1), one would track:
- Safety: hs-CRP (systemic inflammation), Complete Blood Count (neutrophils/lymphocytes), and Lipid Panel(mTOR inhibition can raise lipids).
- Efficacy (Surrogates): Since you cannot biopsy your brain for amyloid, use Cognitive Testing (e.g., CNS Vital Signs) and serum Neurofilament Light Chain (NfL) (a marker of neuronal injury) or p-Tau217 (blood-based Alzheimer’s marker).
Feasibility & ROI
- Sourcing: IMPOSSIBLE for the specific formulation. The FibCS1-PEG-b-PLA micelle is a lab-bench creation.
- Alternative: “Raw” Rapamycin powder dissolved in solvents (DMSO/Ethanol/Saline) is used by some biohackers for intranasal spray. Warning: This lacks the targeting peptide and the micelle stability. It may simply drip down the throat (oral ingestion) or damage nasal mucosa due to solvents.
- Cost: Rapamycin (Sirolimus) is cheap (~$1–$3/mg generic). The cost is negligible; the barrier is the delivery chemistry.