This information came out in December, and was presented on at the Longevity Conference but I haven’t had time to cover it. Here are some details:
The results were discussed at the JP Morgan Biotech Conference a few weeks ago, as covered here: Video: Therapies to Target Muscle Aging (Wednesday, Jan. 11)
At the Longevity Summit Conference BioAge covered some of the muscle results of their BGE-105 Drug that looks as though it will preserve muscle strength during aging (or inactivity). Initially the drug will be targeted at muscle wasting that is common with bed-rest of elderly patients or patients in the ICU.
The broader market is, of course, for people who don’t want to lose muscle strength as they age (despite less activity).
Below are some of the slides that were presented at the Longevity Summit, and a the bottom of this page are details on the Phase 1b clinical trial results:
Full details on this new drug and the results from the Phase 1 B trial are below (from the press release):
BioAge Announces Positive Topline Results for BGE-105 in Phase 1b Clinical Trial Evaluating Muscle Atrophy in Older Volunteers at Bed Rest
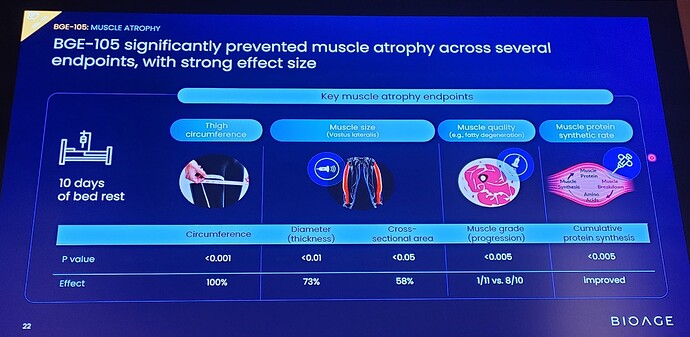
Apelin agonist BGE-105 resulted in statistically significant improvement vs. placebo in muscle size, quality, and protein synthesis in volunteers ≥65 years old during 10 days of bed rest, with no serious adverse effects
Results support advancement to Phase 2 study of BGE-105 to prevent adverse muscle atrophy–related outcomes for older patients in the ICU
December 05, 2022
BioAge Labs, Inc. (“BioAge”), a clinical-stage biotechnology company developing therapeutics that target the molecular causes of aging to extend healthy human lifespan, today announced positive Phase 1b clinical data for BGE-105, a highly selective, potent, orally available small-molecule agonist of the apelin receptor APJ. BGE-105 treatment resulted in statistically significant prevention of muscle atrophy relative to placebo in healthy volunteers aged 65 or older after 10 days of strict bed rest.
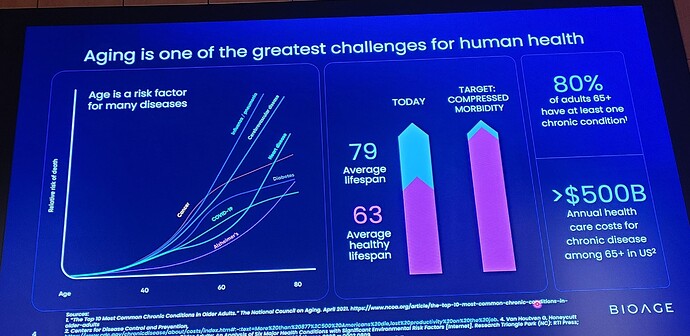
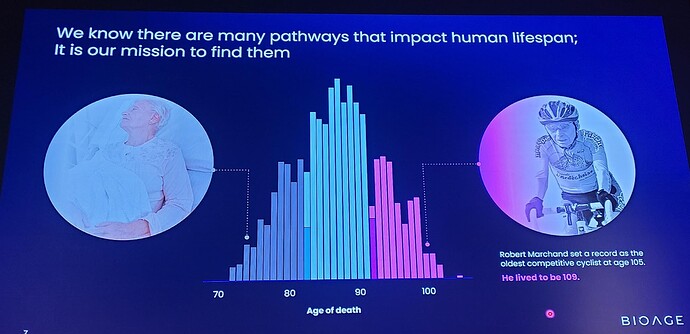
Muscle atrophy—loss of muscle mass and strength—is a universal feature of human aging that increases the risk of multiple morbidities, shortens lifespan, and diminishes quality of life. Hospitalization and periods of forced inactivity greatly accelerate this loss in older people.
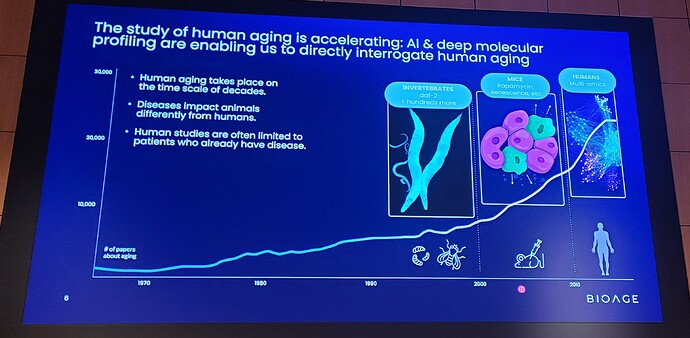
“The data from this Phase 1b study provide clinical validation of BioAge’s AI-driven discovery platform and demonstrate the power of our human-first approach to identify medically relevant drug targets,” said Kristen Fortney, PhD, CEO and co-founder of BioAge. “Our analysis of BioAge’s human aging cohorts revealed that the apelin pathway is a strong predictor of healthy longevity and muscle function, and now this has translated directly into our clinical finding that apelin pathway activation with BGE-105 improves muscle physiology in older adults. Today’s announcement is a milestone in BioAge’s mission to create a pipeline of drugs that treat disease and extend healthy lifespan by targeting the mechanisms of aging.”
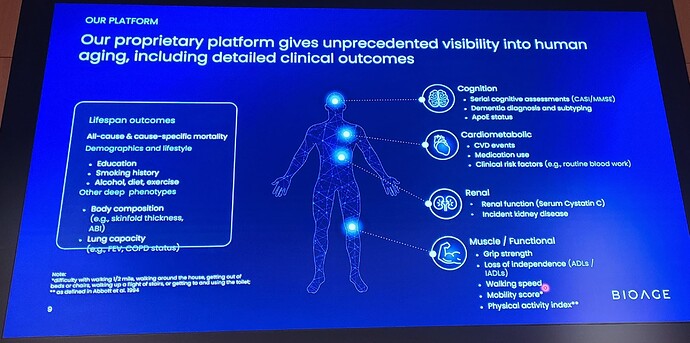
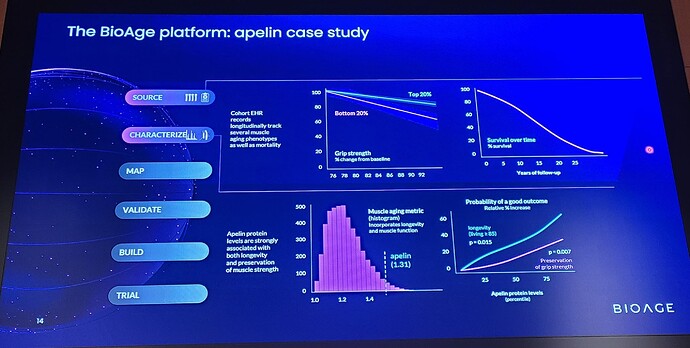
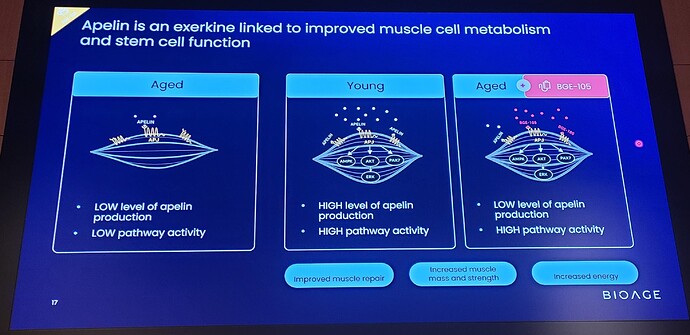
BioAge’s analysis of proprietary human biobanks showed that apelin pathway activity, which declines with age, is positively associated with longevity, mobility, and cognitive function. Apelin, the natural ligand of APJ, is secreted by skeletal muscle in response to exercise and regulates multiple aspects of muscle metabolism, growth, and repair. BGE-105 binds APJ and activates apelin signaling. In April 2021, BioAge entered into an exclusive worldwide license agreement with Amgen, Inc. to develop and commercialize BGE-105 for all indications.
Study design and results
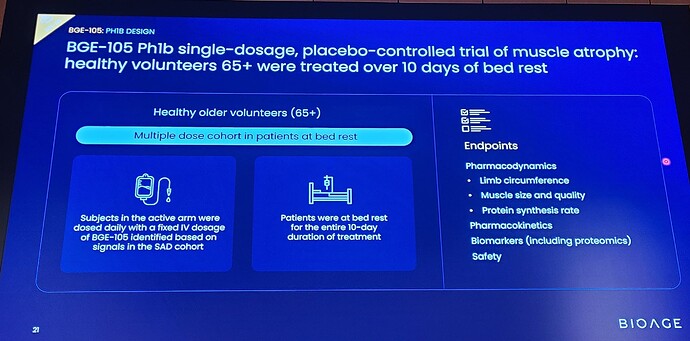
The double-blind, placebo-controlled trial evaluated the safety and pharmacodynamics of BGE-105. Twenty-one volunteers underwent 10 days of bed rest while receiving infusions of BGE-105 or placebo.
Volunteers on placebo (n=10) exhibited muscle atrophy, reflected by statistically significant reductions in thigh circumference and ultrasound measurement of vastus lateralis muscle dimensions (cross sectional area and thickness) and muscle quality (fatty degeneration).
Treatment with BGE-105 (n=11) significantly ameliorated muscle atrophy relative to placebo:
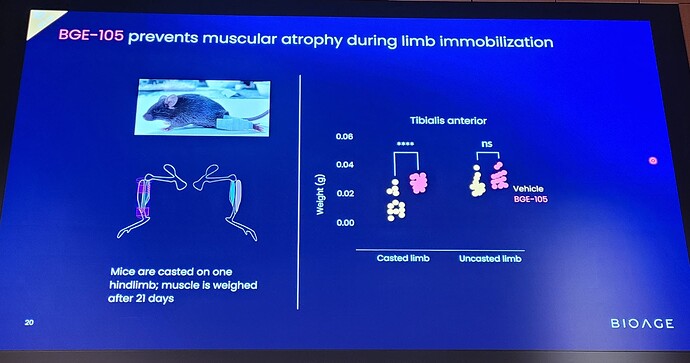
Muscle dimensions: Volunteers receiving BGE-105 showed a 100% improvement in thigh circumference (p < 0.001) relative to placebo-treated volunteers, and ultrasound measurements showed a 58% improvement in vastus lateralis cross-sectional area (p < 0.05) and a 73% improvement in vastus lateralis thickness (p < 0.01).
Muscle quality : Ultrasound echo density measurements revealed that the Goutallier grade, an index that quantifies fatty degeneration in muscle, worsened in 8 of 10 volunteers on placebo vs. only 1 of 11 volunteers receiving BGE-105 (p < 0.005).
Muscle protein synthesis : Proteomic analysis of muscle microbiopsy samples revealed that bed rest decreased production of muscle proteins, and this effect was significantly ameliorated by BGE-105 (p < 0.005). The higher rate of muscle protein synthesis in the drug vs. placebo group provides a potential mechanistic basis for BGE-105’s protective effect on muscle dimensions.
The drug was well tolerated in the study, consistent with prior phase 1 trials conducted by Amgen showing that oral or intravenous BGE-105 was safe and well-tolerated in 198 subjects.
“Older men and women lose substantial muscle mass during periods of immobilization and bed rest, often with devastating consequences,” said William Evans, PhD, Adjunct Professor at UC-Berkeley and Duke University. "Based on the promising data from this Phase 1b study, BGE-105 should be investigated for the treatment of a wide range of age-related syndromes driven by loss of muscle, including acute myopathies in hospitalized patients as well as chronic medical conditions that are common among millions of older people but lack effective therapies, representing an enormous unmet clinical need.”
Plans for advancement to Phase 2
BioAge plans to proceed with a Phase 2 trial of BGE-105 to prevent adverse outcomes in older patients under mechanical ventilation in the intensive care unit (ICU). The study will assess the ability of BGE-105 to prevent two conditions: ICU diaphragmatic atrophy and critical illness myopathy, a broad weakening of the muscles due to disuse under prolonged bed rest. These conditions, which affect millions of patients every year, are both associated with poor clinical outcomes and substantially increased mortality. No effective treatments are currently available, representing a high unmet medical need. The Phase 2 trial is anticipated to begin in 2023. The company will also continue to develop the drug for chronic conditions related to muscle aging.