dan_hayes is leaning hard into cynicism here, but his response does highlight the challenges of dosing rapamycin for longevity. We do not have gold-standard RCTs comparing different dosing schedules of rapamycin, initiated at different ages, and tracked across the duration of every participant’s lives. Nor do we have shorter-term human studies comparing dosing schedules with a more tractable longevity-based readout (e.g. epigenetic age, burden of senescent cells, etc).
Does that mean all we have are “opinions and no proof of anything”? Nah. We can do better than that. Just because we don’t have the best evidence doesn’t mean we have no evidence. In fact, we have quite a lot of evidence: a PubMed search for “rapamycin” returns about 53,900 published papers—including 3,295 clinical trials and 1,991 randomized control trials.
The central challenge is that we have to repurpose the data in these studies, as they generally investigate rapamycin in the context of organ transplants or cancer. Still—the data are there for those willing and able to triangulate it.
The mTORC2 complex plays a central role in glucose homeostasis (Hagiwara et al. 2012), (Yuan et al. 2012), (Lamming et al. 2014), (Lamming et al. 2014b), (Arriola Apelo et al. 2020). Rapamycin’s ability to cause glucose intolerance and insulin resistance stems from its inhibition of mTORC2, not mTORC1 (Lamming et al. 2012). Happily, mTORC2 is insensitive to acute doses of rapamycin. Unhappily, mTORC2 takes a hit if rapamycin is around too long:
chronic exposure to rapamycin, while not affecting pre-existing mTORC2 [complexes], promotes rapamycin inhibition of free mTOR molecules, thus inhibiting the formation of new mTORC2 [complexes] (Sarbassov et al. 2006).
How confident are we that inhibiting mTORC2 is a bad thing?
In sharp contrast to mTORC1, inhibition of mTORC2 has mostly negative effects on lifespan […] Genetic inhibition of mTORC2 in one or more tissues of a mouse can result in frailty, hyperphagia, insulin resistance, hyperlipidemia, hypercholesterolemia, hyperglycemia, kyphosis and/or obesity […] Specific inhibition of mTORC1 in mice… did not result in hyperglycemia, impaired glucose tolerance, hyperlipidemia or hypercholesterolemia, again demonstrating that these negative effects are mediated at least in part by inhibition of mTORC2 (Mannick and Lamming 2023).
mTORC1 inhibitors… but not dual [mTORC1/2] inhibitors protect against inflammation-induced apoptosis, senescence, and matrix catabolism in human disc cells, which depends on Akt and autophagy induction (Kakiuchi et al. 2019).
What do we know about when rapamycin begins affecting mTORC2?
In male and female C57BL/6 mice, 16 weeks of oral rapamycin (2.24 mg/kg body weight/day ≈ 22.7 mg at 75 kg body weight) had no effect on the phosphorylation of Akt (Ser473) and PKCα (Ser657)—both targets of mTORC2—but decreased the phosphorylation of p70S6K (an mTORC1 target) by ~34% in whole brain lysates (Halloran et al. 2012). This indicates that, at least in the brain, concerns over rapamycin’s capacity to suppress of mTORC2 are not warranted.
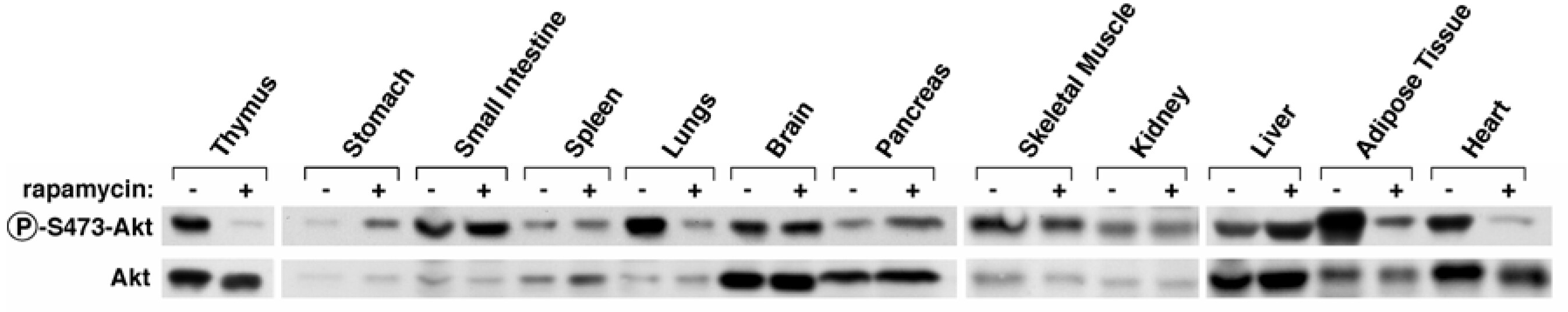
In three-month-old male C57BL/6NTac mice, 7 days of intraperitoneal injection with high-dose rapamycin (10 mg/kg bodyweight ≈ 101.5 mg at 75 kg) inhibited mTORC2 (reduced pAkt/Akt) in the thymus, lungs, adipose tissue, and heart (Sarbassov et al. 2006). Consist with (Halloran et al. 2012), rapamycin did not inhibit mTORC2 in the brain.
Here’s a relevant figure from (Sarbassov et al. 2006). The first row shows the amount of phosphorylated Akt (an mTORC2 substrate). If mTORC2 is inhibited, we will see less phosphorylated Akt. You can see a marked decrease in the thymus, lungs, adipose tissue, and heart—after 7 days of intraperitoneal injection with high-dose rapamycin.
In 26-week-old male psPten–/– mice (n = 15), eight weeks of 3x weekly oral doses of a highly bioavailable nanoformulation of rapamycin called Rapatar appeared to increase the amount of phosphorylated Akt at both a low (~0.1 mg/kg rapamycin) and a high (~0.5 mg/kg rapamycin) dose, but only the high dose was significant (p = 0.02) (Antoch et al. 2020). The increase in pAkt could result from releasing mTORC1’s inhibition of IRS1/2 and mTORC2 (Rozengurt et al. 2014). Direct inhibition of mTORC2 has previously required higher doses: e.g. 10 mg/kg in (Halloran et al. 2012) and 8 mg/kg in (Schreiber et al. 2015). These results indicate that rapamycin causes a compensatory activation of Akt at doses below the threshold of mTORC2 inhibition. I suspect this increase in Akt underlies the mTOR rebound sometimes seen with rapamycin, but that’s a topic for another post.
In (Chen et al. 2010), a low concentration of rapamycin (10 nM) didn’t inhibit mTORC2 after 24 hours, while a high concentration (1000 nM) did. This paper used two different cell lines: DU-145 and MCF-7. We can roughly extrapolate these numbers to circulating levels of rapamycin in ng/mL. Here’s the math:
10 nM = 10/1000/1000/1000 = 0.00000001 M = mol/L
= 0.00000000001 mol/mL x 914.187 g/mol = 0.00000000914187 g/mL
= 9.14 ng/mL
The 1000 nM dose is 100x higher: 914 ng/mL. This gives us one piece of evidence suggesting that 9.14 ng/mL avoids mTORC2 inhibition. I welcome others to find other in vitro or ex vivo studies to add more nuance to the dosage range that avoids inhibiting mTORC2.
What human dosing data do we have?
the maximum-tolerated dose of oral rapamycin administered to adult cancer patients on a daily basis has been reported at ~6 mg/d, which results in a maximal plasma concentration of ~22 nM (Jimeno et al. 2008)
A circulating concentration of 4.74 ng/mL rapamycin showed clinical benefit to patients with familial adenomatous polyposis (Yuksekkaya et al. 2016).
For transplant patients, rapamycin’s therapeutic window is 5 to 15 ng/ml in whole blood (Burke et al. 2022).
Personally, a 12 mg dose of rapamycin taken with food yielded an approximate peak of 17.9 ng/mL three hours later. The blood level (ng/mL) achieved by a given dose (mg) varies widely from person to person, so everyone needs to get their own blood work done.
Takeaways
-
Unless human clinical data eventually says otherwise, design your dosing schedule to avoid inhibiting mTORC2. You’ll need some personal data points to assist with that. A peak rapamycin level of 10 ng/mL probably avoids inhibiting mTORC2.
-
Get three sirolimus/rapamycin blood tests: 3 hours after your dose (~peak), 7 days after your dose (potential trough), and 14 days after your dose (confirming the trough).
-
Design your dosing schedule based on the levels you see at 7 days and 14 days. Based on rapamycin’s half-life, I expect most people to have detectable levels at 7 days. I suspect that matters less for lower doses, as the ng/mL amount of rapamycin would likely be below the mTORC2 inhibition threshold. But for higher doses, 14 days is probably wiser. But personal blood work is necessary to determine those parameters.
-
I’m not currently aware of studies that provide significant insight on the duration of a “rapamycin vacation”. In female C57BL/6NCr mice, 15-months of intraperitoneal injection with 1.5 mg/kg rapamycin, 3 times a week every other week, resulted in sustained mTOR suppression in the heart when measured 13 days after the last dose (Leontieva et al. 2014). The same dose given orally did not have that effect. So, either 13 days was sufficient to restore normal mTOR signaling in mice—or that dose never substantially lowered it to begin with. If anyone is familiar with studies that could shed light on the duration of a dosage break, please join in. As it stands, I don’t think mTORC2 signaling would present an argument for breaks longer than two weeks. But it may turn out to help reduce rapamycin’s other side-effects (e.g. hyperlipidemia). In that case, personal blood work to monitor ApoB would help direct the frequency and duration of rapamycin breaks.
Currently, I prefer an alternating schedule with rapamycin (Week 1), 24-36 hour fast (Week 2), rapamycin (Week 3), 24-36 hour fast (Week 4), break (Week 5), then repeat. But I will update and tweak that based on 1) new evidence and 2) my own blood work.
I didn’t intend to spend the morning writing this, but there you have it! We do not have definitive answers, but we’re miles and miles ahead of having nothing to go on. Bottom Line: We know enough to design more and less rational dosing schedules.