To be honest, I don’t see the value in doing this. I understand if you’re a doctor or a researcher and you have to know as much as possible or fill in some possible knowledge gaps. If you think you’ve found a counter argument to anything said, just bring up that argument and we can discuss it. Asking those you’re debating with to start reading textbooks means you’ve probably run out of arguments. And that’s fine. I already said we don’t have to debate about 70 vs. 40 LDL. As I don’t have any arguments, except extrapolating from short term randomized trials (PCSK9i and statins), mostly.
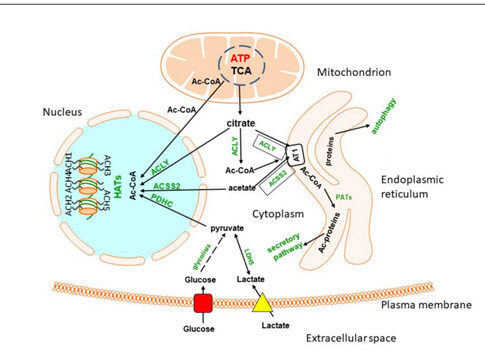
Potentially, but there are many , many ways for acetyl-CoA to be produced outside of the mitochondria. You have the ACSS, ACLY, PDH enzymes. You can have conversion of Acetate, lactate, NAA, glucose, pyruvate, citrate, etc.
Also those acetyl-CoA are not being used purely for cholesterol(as I am sure you are aware), they play a role in other things outside of lipid synthesis. It is also used for hundreds of acetylation reactions, including N-acetyl aspartate synthesis in neuronal mitochondria, acetylcholine synthesis in cholinergic neurons, as well as divergent acetylation of several proteins, peptides, histones and low-molecular-weight species in all cellular compartments.
Here is a good picture of some of the potential pathways for Acetyl-CoA( I study ACSS2 function in the brain).
Another thing to consider is where in the body acetyl-CoA levels increase. In the brain, it gets complex.
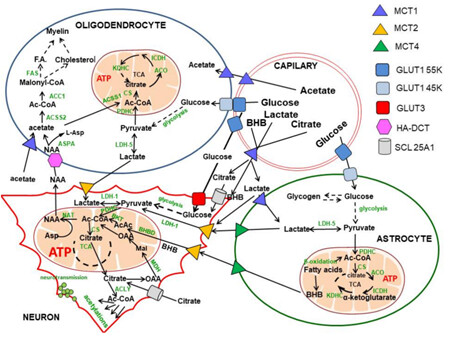
Point is, there are probably other factors involved in the increase of cholesterol we see with Rapamycin.
ACLY, however, is fed by the non-canonical TCA cycle which is where mitochondrial health comes in. From the perspective of histone acetylation ACCS2 is interesting in that it is inhibited by growing levels of acetylation, hence if acetates increase acetylation will only go so far.
The reason I am particularly interested in Acetyl-CoA metabolism is that I believe this is at the core of two aspects of the key aging pathway which results in longer genes being expressed less over time. I wrote a blog post about this with links to the relevant research which is here:
In your studying about acetyl-CoA levels in the brain you may wish to look at my own experience. My biohacking experiments are designed to increase cellular acetyl-CoA (primarily to prevent the stalling of RNA Pol II and consequential aberrant RNA splicing). I am doing this on a cyclical manner where I intend to increase it in the morning starting roughly when I get up at 6am and stopping perhaps about 5pm, but obviously things go down more slowly than that. I do think it affects sleep in that if I stop the processes later in the day it is disruptive to my sleep.
The logic of this is by providing the substrate for HAT/KAT as part of the RNA Pol II complex there is less stalling. The macroscopic results are changes in biomarkers and the growth of new hair in various places as well as changes to some of the indicators of aging. The side effects that occur are the side effects that one would expect from providing more cytosolic Acetyl-CoA including a temporary increase in cholesterol although I have now driven down my LDL cholesterol levels although initially they were higher (although there are lots of problems with labs in measuring cholesterol). Also the availability of substrate for cytosolic ROS creation can result in inflammation although I have found it quite good at fighting infection and I think that in part is the cause of my WBC being low. Rapa pushes it down further, but I take Rapa quite infrequently.
Another interesting thought I have just had based upon this conversation is that another thing that disrupts my sleep is menaquinone-7. (Vitamin K2 MK7). Now this molecule is interesting because it provides an additional electron transport for the mitochondria. Papers seem to indicate that it increases ATP production through OxPhos by about 5%, but I wonder whether that additional available energy has similar effects to the additional Acetyl-CoA. Whichever way additional energy tends to disrupt sleep. It may be that some of the additional Acetyl-CoA feeds back into the mitochondria to generate more ATP.
Correct on ACLY being fed non-canonical TCA cycle, but it also can take citrate produced through the TCA cycle and utilize that for Acetyl-CoA production, it can do the same with acetate. ACSS2 also recycles acetate that are released from HDAC reactions, allowing it to be used for Acetyl-CoA.
My focus has been on ACSS2 role in neuropsychiatric disorders and sleep. I recently conducted some experiments showing that inhibiting ACSS2 promotes resilience in social defeat stress model. My next step is to see how alterations in ACSS2 impact sleep homeostasis. I would imagine that changes in intracellular Acetyl-CoA levels will impact sleep homeostasis, since alot of our circadian clock genes are regulated through these epigenetic modifications, along with the fact you would get changes in sleep promoting gene transcription. Alterations in intracellular Acetyl-CoA pools have been shown in numerous neuropsychiatric disorders, as acetylation is a key regulator in immediate early gene function and neuronal plasticity and long term memory. I would imagine that too much acetyl-CoA will cause over transcription of genes, where some of those genes may be good, but others may not.
But if you want to talk about this more, we can have private message discussion or move this to another topic in the forum, because I think we are straying away from the main topic of focus.
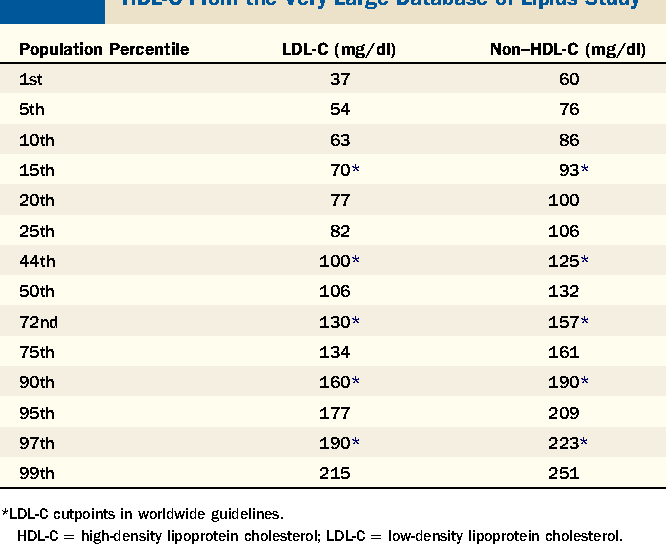
Non HDL is emerging as perhaps the most important lipid measurement. Here’s a very nice summary and suggests a non hdl of < 130.
The most important sentence is this, “ that said, it’s important to consider risk factors for cardiovascular disease on an individual basis rather than rely on the numbers.”
For the non clinician, it’s simpler to just stare at the numbers , but I suspect that the majority of rapamycin users are low risk and don’t need to jump to statins.
For those still concerned, bergamot is an excellent alternative
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4702027/
That is around the 50th percentile. I don’t think that is low enough as the average Joe or Jane gets heart disease. Under the 10th percentile would be 86 and under the fifth percentile below 76. There is plenty of benefit from going lower than the median.
I think levels around the normal levels of Acetyl-CoA tend not to have that much non-enzymatic acetylation.
This applies to so many more situations than just alternative medicine. Political wackos in both parties, conspiracy theorists, etcetera . . . they all enjoy feeling “special” because they know something big that others don’t, and that shadowy “others” are trying to cover up. (Big Pharma, the Deep State, whatever.)
Personally I think that most of them just have boring, disappointing lives, and they really need something that makes them feel more important and powerful.
CAD is not the only cardiovascular disease of aging. Another rather significant issue is cardiac arrhythmia, the most common of which is atrial fibrillation. This happens when the atria begin to contract in a very irregular manner and can lead to various problems including rapid heart rate, heart failure, fatigue,reduction of exercise capacity, and most seriously, stroke.
I discussed this issue yesterday with a cardiologist who is board certified in 4 sub areas of cardiology. His belief is that if we live long enough, say 100+, we’re basically all at great risk of getting A fib because it’s essentially a disease of aging.
Given enough time, the sinus node just wears out, and fortunately the heart is able to compensate by having other areas of the heart initiate heart beats. The fibrillation, however, allows for blood clotting, and these can then go to the brain resulting in strokes. These type of strokes tend to be particularly deadly.
So if A fib is a disease of aging, will rapamycin prevent it, or at least greatly delay it? Is it a product of senescence? Hyperfunction?
It’s a common and serious cardiac disease of aging, and I hope that rapamycin is protective.
My hypothesis is that aging is a problem of the failure of gene expression for longer genes. A fib is probably part of this. Hence rapamycin probably helps. (as do other approaches)
Rapamycin does indeed lead to increased gene expression of the RAD protein which is protective against cardiac hypertrophy. Left atrial enlargement is a known risk for cardiac arrhythmias and stroke.
This is a study on a particular strain of mice that are actually prone to cardiovascular disease, similar to humans.
Rapamycin improved cardiac contractility, inflammation, and mitochondrial function, while directly preventing against cardiac enlargement.
I’ve repeatedly made the point in this thread that statins should be reserved for those at higher risk for cardiovascular events. They’re not indicated for the low risk patient. It can, however, be difficult to determine degree of risk. This article in The Journal of Clinically Lipidology makes a strong case on basing these decisions on the coronary calcium score.
In summary, for those of a score of zero, or 1-99, statins aren’t indicated.
For a score >100 , a moderate intensity statin is in order.
For a score > 300, a higher intensity statin is indicated, or moderate statins with combination therapy.
Of interest, even with an LDL > 190, many of these patients have a CAC of zero and don’t require statins, but repeated scores are in order.
What about Zeta and PCSK9i? Same thing?
It’s all about benefit/ risk, so yeah, same thing. Recommendations for all of the cholesterol lowering drugs is based on risk stratification since all clinicians are aware of long term medication risks.
However, there’s certainly no reason to not use very safe interventions like oats, fiber, flax, and even bergamot.
What about ubiquinol or CoQ10?
Rapamycin slows the progression of atherosclerosis by attenuating the recruitment of macrophages:
After listening to When CAC Tests Are Useful and When They Are Not | The Peter Attia Drive Podcast - YouTube , I’m not convinced that CAC scores are an indicator of risk, especially for those interested in longevity. In older patient, a nonzero score doesn’t tell you much, because we expect it from aging. And in younger ones, a CAC score of zero doesn’t tell you much either since your apoB could be abnormally high. CAC score of zero doesn’t imply that there has been no arterial damage. You can still have soft plaque that hasn’t been calcified yet.
My conclusion is that if you’re interested in longevity, CAC scores are at best useless. We’re looking way past 10-year risk numbers, we’re more interested in 30-50 risk numbers.
The whole Attia podcast is worth a listen: Preventing Atherosclerosis: 2 Flaws with the 10 Year Risk Approach | The Peter Attia Drive Podcast - YouTube
It’s an extremely valuable prognostic indicator even at 15 years out:
https://www.ahajournals.org/doi/full/10.1161/CIRCIMAGING.115.003742
Of course it would be better if we had 50 year studies.