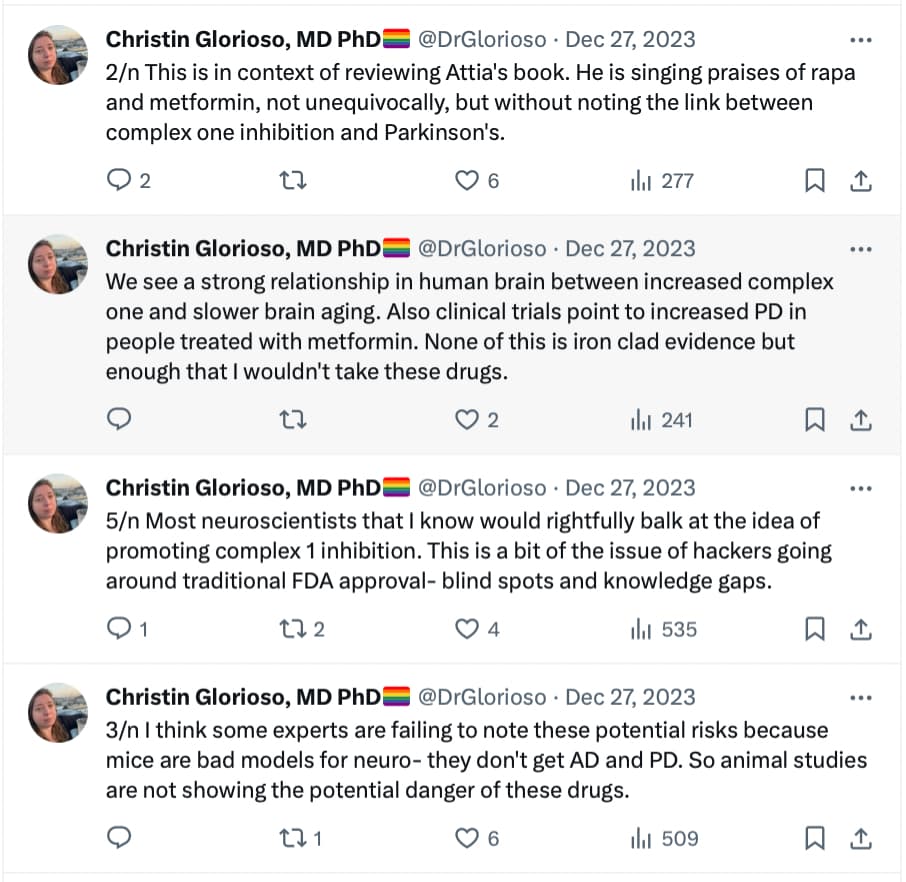
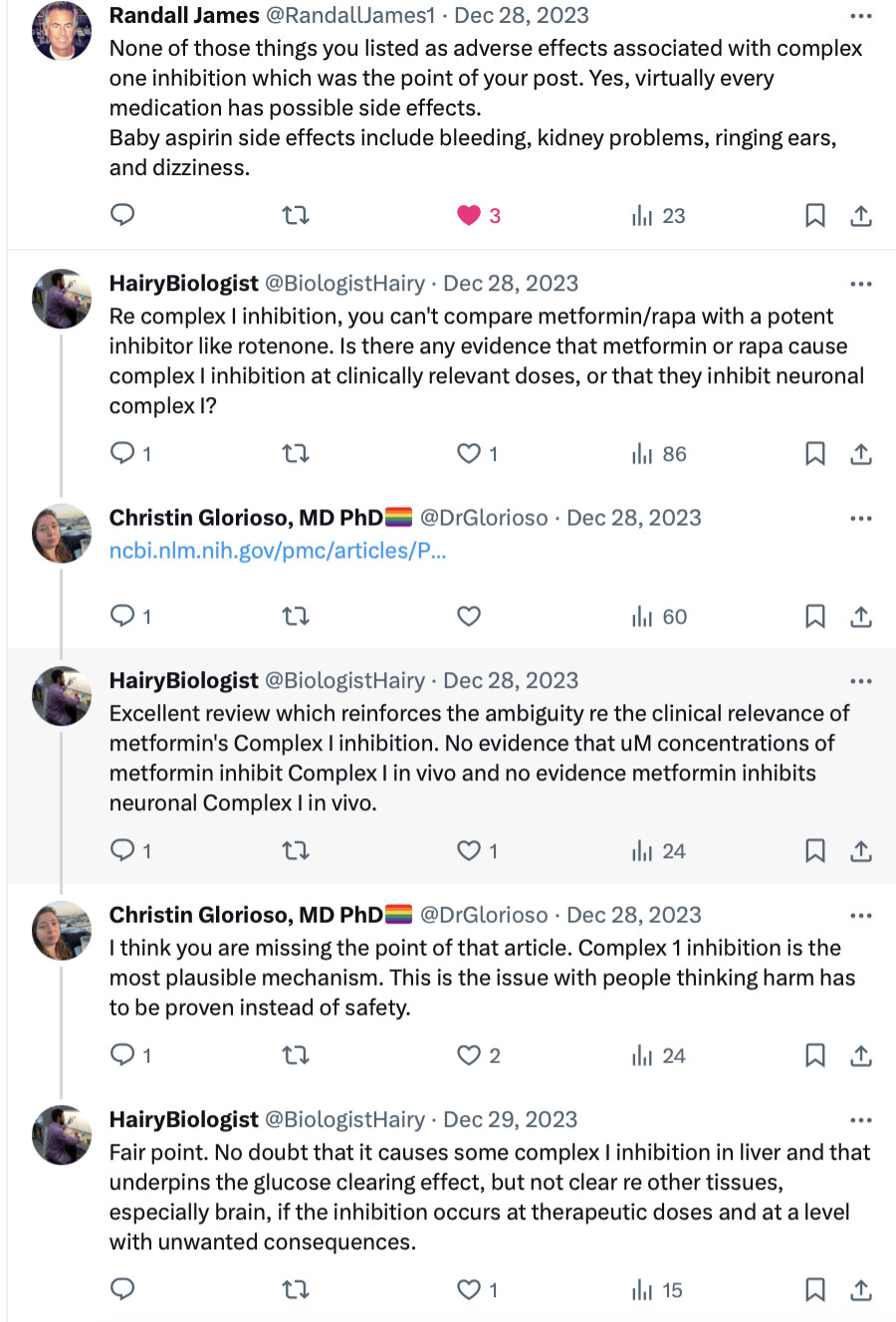
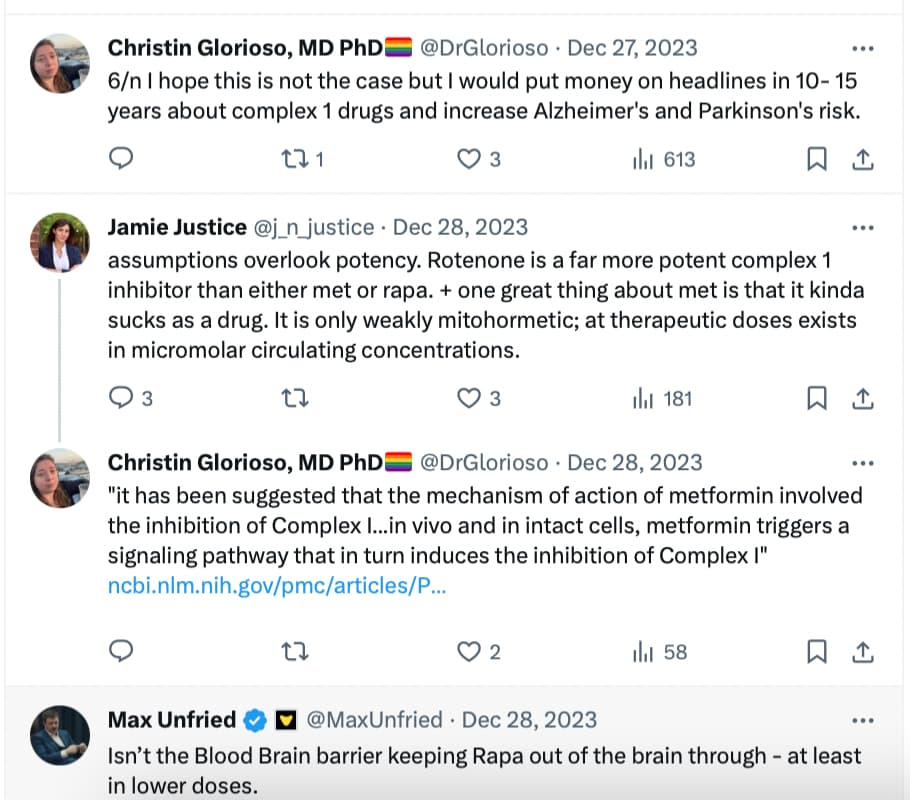
The link between PD and metformin has been detected long ago. See, for instance: RENEWAL: REpurposing study to find NEW compounds with Activity for Lewy body dementia—an international Delphi consensus 2022
Meanwhile, several epidemiological studies have found associations between use of metformin and lower incidence of either all-cause dementia, AD or PD [152,153,154,155,156,157]. However, not all epidemiological studies have found associations between the use of metformin and a lower risk of dementia [158,159,160]. A study using the National Alzheimer’s Co-ordinating Center database investigated the effect of oral hypoglycaemic drugs on longitudinal memory decline among patients with T2DM with either normal cognition (n =1192) or with AD (n =807) and found that metformin was associated with better memory performance in non-demented participants (mean duration of follow-up 3.4 years) but it had no effects in AD (mean duration of follow-up 1.9 years) [161]. A systematic review and meta-analysis of observational studies testing the association between metformin and neurodegenerative diseases analysing a total of 19 studies with 285,966 participants found no association between metformin exposure and incidence of all subtypes of neurodegenerative diseases, and found that metformin monotherapy was associated with an increased incidence of PD compared to non-metformin or glitazone users (OR 1.66) [162].
The reason for the discrepancy between studies (besides the difficulty to separate the effects of diabetes from the effects of the drugs, but with a lot of data, comparison to other diabetic drugs, and genetic mendelian randomization, researchers now know how to do that properly) is probably that the effects of metformin seem dose-dependent (“The dose makes the poison” as the saying goes…): Dose–Response Association of Metformin with Parkinson’s Disease Odds in Type 2 Diabetes Mellitus 2022
Metformin was associated with PD odds in T2DM in a dose–response association manner. Patients who received low dosage and intensity of metformin use were associated with lower odds of PD, while higher dosage and intensity of metformin use had no neuroprotective effect.
What they call “low-dose” in the paper is <10 DDD/month so < 20 g/month, meaning < 0.650 g/d. But they also say that you shouldn’t take more than 300 cDDD = 600 g ⇒ 0.550g/d over 3y or 0.3g/d over 5y (as the study looked at people over a follow-up period of 3 or 5 years).
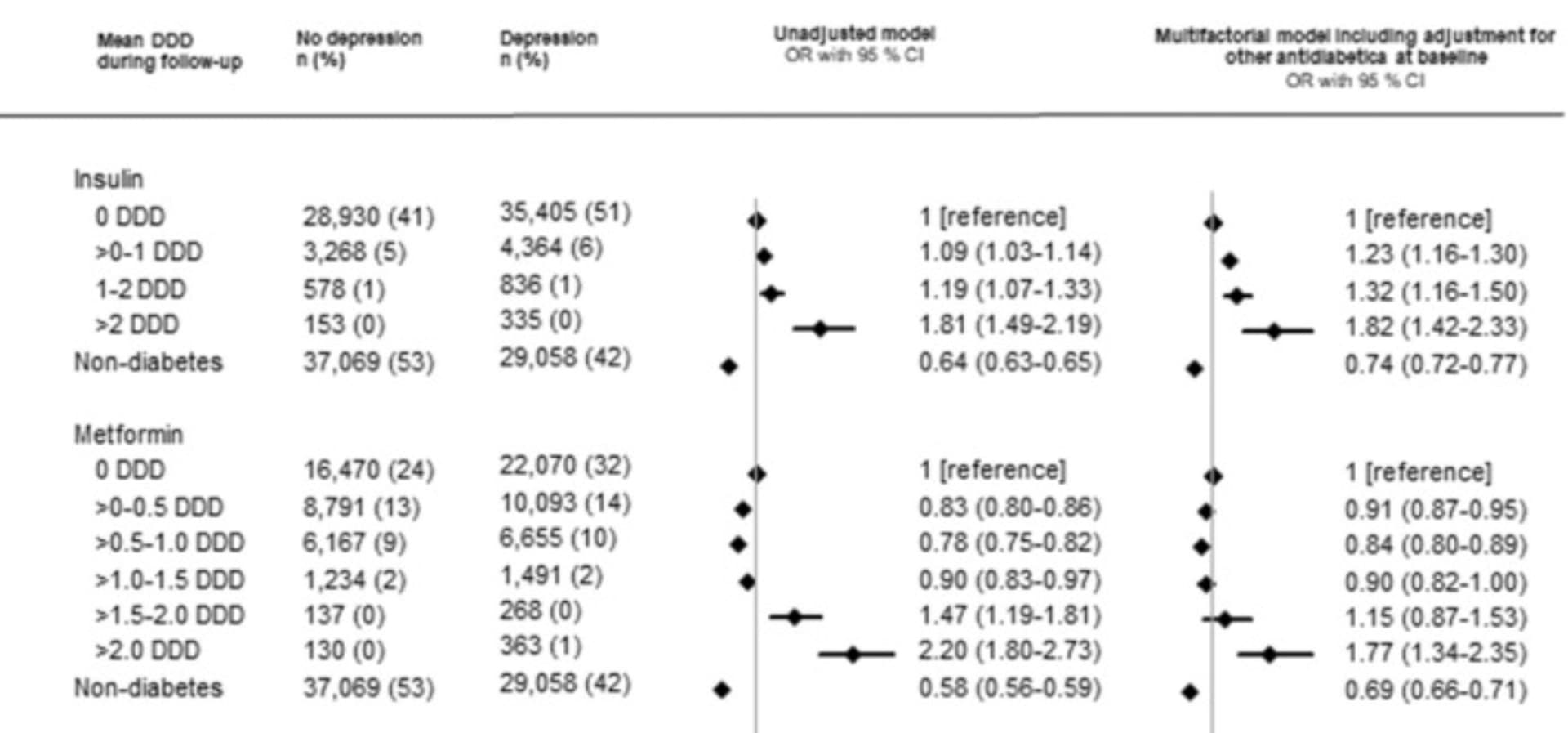
You find the same dose-dependent relationship between metformin and depression (and depression is often an early symptom of Parkinson’s / "pre-Parkinson’s), but with slightly higher thresholds (the optimal here is 0.5–1.0 DDD so 1 to 2g/day of metformin): Diabetes, antidiabetic medications and risk of depression – A population-based cohort and nested case-control study 2022
So yes, the risk is well known. I would not take metformin if I had any family history of Parkinson’s disease (or related genes). Especially as we know more potent and safer alternatives. GLP1 agonists and SGLT2 inhibitors are associated with lower rates of AD, PD, other dementia, and depression in most longitudinal studies on humans:
These findings were confirmed in several rodent models, for instance:
Even better, we have three good-quality phase 2 clinical trials showing that 3 different GLP1 agonists (exenatide, liraglutide, and lixisenatide) slow down the progression of Parkinson’s disease. And one clinical trial showed that empagliflozin (SGLT2i) improved MCI: Empagliflozin Improves Cognitive Impairment in Frail Older Adults With Type 2 Diabetes and Heart Failure With Preserved Ejection Fraction 2022
I don’t know about rapamycin but there are two trials for AD (NCT04200911, phase 1 done, and NCT04629495, ongoing phase 2), both in Texas). And rapamycin was shown to have no effect (neither positive nor negative) on MSA: mTOR Inhibition with Sirolimus in Multiple System Atrophy: A Randomized, Double-Blind, Placebo-Controlled Futility Trial and 1-Year Biomarker Longitudinal Analysis - PubMed
So the jury is still out on rapa for NDD… (my two cents: given that diabetes is protective of PD and that rapamycin increases Hb A1c, I would not be surprised if rapamycin, alone or in combo with a GLP1RA or SGLT2i, would be highly protective of PD, but that’s a pure guess…)