What are your thoughts about the potential for mTOR2 inhibition?
Amazing overview… because of you MAC I am doing a lot more testing and liking the information it provides… flying a little less blind. DEXA, Coronary Calcium Scan, Labcorp sirolimus trough and post dose levels with GFJ… and without , ApoB… etc.
I highly recommend everyone take your lead and do the testing - look under the hood.
Thanks buddy!!
Somatropin is just one of the cheaper variants of HGH. However, HGH itself isn’t anything new. It’s been used in various therapies for decades, particularly for teens that have low growth hormone (the most famous example being Lionel Messi).
It has a lot of pretty nasty side effects, though, most notably its diabetogenic effect. It’s also something that seems to accelerate aging when tested in various animal models. This doesn’t matter as much to bodybuilders, but to people who want longer lifespan in addition to healthspan, it’s not ideal.
that person who went to the hospital likely did not need to. eventually the infection would have subsided as mine did after using tap water in philippines which i thought was ok but now know it likely was not since i got bad diarhhea from just drinking it for the first time in another place. i always used sterile water at home but did not want to have to take extra weight of baggage so now just use boiled i assume drinking water in PI which most all locals only drink also.
yea i was doing hgh but due to the high cost years ago switched to mk677 which can be taken orally as i do about 20mg/day and much cheaper than hgh.
WHAT THE FUCK! You injected using goddamned tap water? In the Phillippines!?!? How are you even alive? Do you even have the limb you injected into?
And you even got an infection? Did you even go to the damn hospital? What do you mean by “subsided?” Did you literally just wait it out?
For the love of all that is holy, please STOP. You are fucking scaring me. Get help, now.
I’m about to faint.
How many drugs are you pumping into yourself? I’ve followed your posts across several threads, and each additional post you make is somehow more terrifying than the last.
I am normally for harm reduction, but at this point I don’t even think you are mentally sound enough to even listen to what I have to say.
I am not exaggerating when I say this. In all my time in pharma, research, and clinical laboratories, I have never seen a patient as reckless, self-endangering, and absolutely insane as you.
Please, please, please seek medical counsel. You are killing yourself. You’re going to die if you continue this behavior. Get off this forum and go to a doctor!
ofcourse i don’t go to any hospital and ofcourse i just wait it out ! oh like anyone who ever gets infection goes to the hospital. you say the most ignorant things ! And i sure as hell would never go to any doctor unless i have a reason ! specifically if he can do the surgery i want which is something i can’t do myself.
@MAC I found this statement in the recent paper by Randy Strong and Dave Sharp interesting… and you might also.
Another major failure of mTOR inhibition by rapamycin was a trial studying intravitreal sirolimus in age-related macular degeneration (AMD) (Petrou et al., 2014). This was unexpected since hyperactivated mTORC1 was widely viewed as playing a major role in development and progression of AMD (Go et al., 2020; Huang et al., 2019; Kaur et al., 2018; Zhang et al., 2020; Zhao et al., 2011; Zigler et al., 2011). Why an intravitreal approach lead to adverse events is somewhat mysterious since systemic sirolimus cured AMD-like symptoms in a mouse model when administer iP (Zhao et al., 2011). In hyperglycemic rats, an IP injection of rapamycin reduced diabetes -induced VEGF overexpression that controls vascular permeability and angiogenesis (Kida et al., 2021). One wonders if systemic intervention with mTORC1 inhibitors would have better success in prevention or treatment in people. It is also noteworthy that the use of mTOR inhibitors in diseases not associated with aging is increasingly wide spread (summarized in Johnson et al., 2013b).
From this paper:
Great info, thanks. Wonder how the gutbiome will change from the acarbose when that time comes ![]()
Strong and Sharp grossly misinterpreted the adverse outcome of this trial to solely “sirolimus”.
The referenced paper on AMD:
https://iovs.arvojournals.org/article.aspx?articleid=2212763
Notable comments from this study:
“We found that intravitreal sirolimus was not associated with systemic safety issues in participants with GA. Systemic exposure to locally delivered sirolimus was below 2 ng/mL, and well below the therapeutic range for systemic immunosuppression. The effect of the investigational agent on the development of choroidal neovascularization cannot be discerned. How sirolimus may be related to the observed effects in treated eyes with GA is unclear”
Intravitreal administration has some major intrinsic risks.
“Endophthalmitis or a bacterial infection within the eye causing inflammation of the sclera, is one of the most severe complications due to intravitreal injections. Another complication of intravitreal medication administration is inflammation. Intraocular inflammation is one of the main causes of temporary pain and vision loss after an intravitreal injection. Severe inflammation can cause permanent damage to the eye. A growing body of evidence has shown repeat intravitreal injections have their own increased risks and complications. A 3x rise in intraocular pressure after an intravitreal injection is expected”
@rapAdmin if you’re trying to associate this singular study negative outcome to a larger pan- parenteral route of delivery for Sirolimus, I don’t believe it’s warranted.
As I review my almost benign alteration to my lipids and glucose on 6 months of therapeutic dosing of rapamycin via IM+IN, it further reinforces my original thesis that it is a superior method of delivering sirolimus with fewer side effects to oral. The following pharmacological model for consideration:
The parenteral route ensures three things primarily:
- Increased bioavailabilty (effectively 100%), avoiding destruction by bile acids
- Avoiding first-pass metabolism in the liver, which further increases bioavailability
- A more sustained-release dose rather than a quick spike from digesting a pill
On the surface, one might think parenteral probably wouldn’t change the dysregulation pathway much (lipids/glucose) since the effective dose is similar to clinical use of rapamycin, namely daily oral dosing to achieve similar trough levels (5-15 ng/mL)
HOWEVER.… it could also be that by mostly avoiding liver metabolism, one is actually reducing the impact of glucose dysregulation on your body, and that levels should have been much more dysregulated had one taken an equivalent dose orally instead of parenterally.
This is because the liver is the primary regulator of glucose metabolism. In traditional pills, after passing through the gastric system, what remains will proceed to be processed by the liver. This means that the liver actually receives the highest dose first, and the rest of the body receives a relatively smaller amount once the liver’s done processing it. By using a parenteral route, one avoids dumping such a high load on your liver, and thus avoid impacting its glucose metabolism as much. This huge fundamental pathway difference might very simply explain my benign labs dysregulation, which should have been massively dysregulated given my clinical level dose and essentially zero symptomatic side effects (fatigue, diarrhea, canker sores, etc).
Furthermore, and another potential huge benefit, higher bioavailability means more drug to diffuse throughout your tissues, potentially forcing some through the blood-brain barrier where lower doses would normally not make it through.
I am currently completing a washout set of expanded labs (oxLDL, FFA, full NMR lipoprotein profile), including a new gut biome kit https://static1.squarespace.com/static/5e8cc993f9169c7045248796/t/62d752c25f3a970effdc4c44/1658278595386/BiomeFx+Sample+Report_July+2022.pdf, and then reviewing the dosing protocol on restart.
Not at all - quite the opposite. My focus was on this: One wonders if systemic intervention with mTORC1 inhibitors would have better success in prevention or treatment in people
I’m becoming more convinced (as do others it seems) that the future for rapamycin is likely injectables!
This paper seems on the surface a massive “promo piece” for eRapa. The authors are massively conflicted.
“The University of Texas Health Science Center at San Antonio has been awarded a patent, U.S. Patent Application No. 13/128,800, by inventors Zelton Dave Sharp and Randy Strong, for an encapsulated rapamycin formulation used in this paper.”
I also take issue with their model of rapamycin and it’s impact on hallmarks of aging, especially cancer. I assume they are implicitly referencing prevention vs treatment. It’s interesting they call out “adult cancer” and not cancer in general. I guess the general thinking is we’re not going to give children rapamycin prophylactically as a longevity intervention.
A review of the HUMAN literature on sirolimus use (therapeutic dosing) is not so cut and dry like many of the cancer prone murine models.
Can Rapamune cause cancer?
“Although rare, it’s possible. Rapamune has a boxed warning Trusted Source for increased risk of certain cancers, as well as infections, due to a weakened immune system. A boxed warning is the most serious warning from the Food and Drug Administration (FDA). Studies of Rapamune reported a few instances of lymphoma (cancer of the lymph system) and skin cancer occurring.”
https://labeling.pfizer.com/showlabeling.aspx?id=139
“Increased susceptibility to infection and the possible development of lymphoma and other malignancies (particularly of the skin) may result from immunosuppression (5.1). Only physicians experienced in immunosuppressive therapy and management of renal transplant patients should use Rapamune for prophylaxis of organ rejection in patients receiving renal transplants.
“13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies were conducted in mice and rats. In an 86-week female mouse study at sirolimus doses 30 to 120 times higher than the 2 mg daily clinical dose (adjusted for body surface area), there was a statistically significant increase in malignant lymphoma at all dose levels compared with controls. In a second mouse study at dosages that were approximately 3 to 16 times the clinical dose (adjusted for body surface area), hepatocellular adenoma and carcinoma in males were considered sirolimus-related. In the 104-week rat study at dosages equal to or lower than the clinical dose of 2 mg daily (adjusted for body surface area), there were no significant findings.”
Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data (2014)
“To examine risk of malignancy and death in patients with kidney transplant who receive the immunosuppressive drug sirolimus. The search yielded 2365 unique citations. Patient level data were available from 5876 patients from 21 randomized trials. Sirolimus was associated with an increased risk of death (1.43, 1.21 to 1.71) compared with controls. Given the risk of mortality, however, the use of this drug does not seem warranted for most patients with kidney transplant. Further research is needed to determine if different populations, such as those at high risk of cancer, might benefit from sirolimus”
Sirolimus effects on cancer incidence after kidney transplantation: a meta-analysis (2015)
“Twenty RCTs and two observational studies were eligible for meta-analysis, including 39,039 kidney recipients overall. After excluding NMSCs, there was no overall association between sirolimus and incidence of other cancers (IRR = 1.06, 95% CI = 0.69–1.63). However, sirolimus use had associations with lower kidney cancer incidence (IRR = 0.40, 95% CI = 0.20–0.81), and higher prostate cancer incidence (IRR = 1.85, 95% CI = 1.17–2.91). A decrease in the overall cancer incidence with use of Sirolimus [outcomes improve to ~26% decrease in cancer rates when prostate cancer was excluded], suggesting that Sirolimus use may be beneficial in the “high cancer-risk” patients”
“This data is exemplified in our Kaplan–Meier survival curves which show that 10–12 years following transplantation, cancer-free survival is improved in patients maintained on Sirolimus. It should be noted that patient survival is equivalent for this length of time, but cancer-related mortality is increased in patients maintained on steroids when compared with sirolimus patients [Table 3]. Therefore, the effects of Sirolimus use may compound over time, allowing for improved cancer-free survival but also delaying the development of cancer and improving cancer-related outcomes. Given these findings, we may begin to see improvements in patient survival 15 and 20 years following initiation of Sirolimus compared to their steroid-based cohorts. Finally, previous studies have determined that sirolimus-based regimens are associated with increased patient, non-cancer mortality [32]. The increased risk of mortality on Sirolimus-exposed patients [HR 1.4–1.7] appears to have resulted from increases in cardiovascular risk as well as infectious complications. An inherent weakness of our study is the use of a historical control cohort consisting of patients transplanted in 2002 and maintained on chronic steroids. It is possible that the differences in outcomes from our two groups may be unrelated to either Sirolimus or steroid exposure over time”
Sirolimus use improves cancer-free survival following transplantation: A single center 12-year analysis (2020)
“From 2003 to 2015, 563 kidney transplant recipient had steroids discontinued by post-operatively and maintained on Sirolimus, Tacrolimus [Tac], and Mycophenolate Mofetil [MMF]. We compared cancer-related outcomes with that of our 65 historical controls (chronic steroids) maintained on Tac, MMF and steroids. Maintenance immunosuppression protocols were Sirolimus (8–10 ng/ml, 24h trough on daily dosing). Patients maintained on chronic immunosuppression with Sirolimus developed statistically-significant lower levels of post-transplant lymphoproliferative disease (PTLD, 5.88% vs. 0.5%; p < 0.05) with no difference in rates of other post-transplant malignancies. Cancer-free survival as well as cancer-free mortality were also improved 10–12 years post-transplant (p = 0.05, p < 0.05). However, long-term patient survival was equivalent in both cohorts (p = 0.22). Patients were followed up to 12 years post-transplantation and monitored for new cancer diagnoses. For skin, breast, cervical, urothelial, and prostate cancers, the prevalence of post-transplant diagnoses were statistically equivalent between the standard [steroid-based] and SBP groups (p > 0.05). The greatest difference in post-transplant cancer diagnoses between the groups was observed in the PROSTATE cancer category, with an increase in diagnoses within the sirolimus group (0% vs.1.76% p = 0.27, Table 3). There is some precedence in the literature for reporting increased risks of prostate cancer in sirolimus-based immunosuppression regimens at 6 months to 5 years post-transplantation [10,11]. While our data shows increased prostate cancer diagnoses in the sirolimus group, this difference is not statistically significant 12 years post-transplantation. However, our 12-year follow-up data did demonstrate a statistically significant difference in the rates of post-transplant lymphoproliferative disorder (PTLD) in the control [steroid-based] vs. SBP groups (5.88% vs. 0.5%, respectively, p < 0.05, Table 3). Additionally, the rate of cancer-related mortality in the sirolimus-based group was also found to be significantly lower (2.94% vs. 0.025%, p = 0.01).”
But are transplant studies good for HEALTHY persons translation???
Regulatory T cells and cancer: an undesired tolerance (2014)
“The incidence of cancer is markedly increased in organ transplant patients. An excess risk of cancer is constantly observed after solid-organ transplantation. A cancer rate similar to that of people 20–30 years older without transplants portrays the importance of the problem. A cancer-related immune phenotype is associated with kidney transplant recipients. More precisely, regulatory T cell (Treg) expansion, a lower B-cell proportion with a larger sub-population of memory B cells, and a higher proportion of CD3+ γδ T cells in cancer patients. Treg expansion had not only the best predictive value for cancer occurrence but also predicted cancer recurrence, correlated with histological grading, and reversed after cancer resection. Although these results have important implications for monitoring and therapeutics, they also raise several questions concerning the nature of these cells, their related prognosis, the effects of immunosuppressive drugs, and subsequent implications for treatment.”
Cancer Risks in Solid Organ Transplant Recipients: Results from a Comprehensive Analysis of 72 Cohort Studies (2020)
“Compared with the general population, solid organ transplant recipients displayed a 2.68-fold cancer risk standard incident ratio (SIR 2.68); renal transplant recipients displayed a 2.56-fold cancer risk, liver transplant recipients displayed a 2.45-fold cancer risk, heart and/or lung transplant recipients displayed a 3.72-fold cancer risk. The increased cancer risk of solid organ transplant recipients is associated with tumour mutation burden, suggesting that iatrogenic immunosuppression may contribute to the increased cancer risk in transplant recipients.”
Organ Transplant and Skin Cancer Risk
“Organ transplant patients (MAC: on chronic sirolimus) are at a higher risk — up to a 100-fold higher — for developing skin cancer compared to the general population. Transplant patients tend to develop a skin cancer called squamous cell carcinoma and Kaposi sarcoma. Many patients also develop a skin cancer called basal cell carcinoma and melanoma. This higher risk is caused by IMMUNOSUPPRESSIVE medications, which are essential to transplant patients to prevent graft rejection and optimize graft survival. Because these medications suppress the immune system that fights off infection and prevents the development of cancer, transplant recipients are at elevated risk for infection and CERTAIN CANCERS.”
These real world human clinical data are quite sobering. Even long term THERAPEUTIC dosing Sirolimus use (10X+ higher than typical longevity rapamycin users 5 mg/week +/-) is not associated with significant improvement in cancer rates reduction (in transplant cohorts), all cause mortality reduction, although lower rates of certain cancer specific mortality, but higher than others?
But do these trials patients TRULY represent good “healthy” controls? It’s impossible to tease out, as immunosuppression is a fundamental treatment paradigm. But then, why would we expect HEALTHY persons taking chronic therapeutic dosing to have different cancer rates IF the immunosuppression is the underlying reason for the INCREASED cancer burden?
“Immunosuppressive drugs have the potential to cause immunodeficiency, which can increase susceptibility to opportunistic infection and DECREASE cancer immunosurveillance”
Sirolimus after kidney transplantation (2014)
https://www.researchgate.net/publication/268879875_Sirolimus_after_kidney_transplantation
“An uncertain future for sirolimus and other mTOR inhibitors The risk of cancer is increased after kidney transplantation and is predominantly attributed to oncogenic immunosuppression. It is a leading cause of death for kidney allograft recipients, and analyses of transplant registry data suggest that mortality related to cancer is on the increase”
The human clinical data does not appear to fully support the mice rapamycin longevity data translation, but MANY confounders. And when one additionally factors the far lower intermittent weekly doses, the absolute translation benefit becomes quite difficult to quantify as it relates to cancer (the mice longevity data we are using for translation).
In this washout period, immunosuppression is probably my biggest concern on restart. One thought is to restart with similar very high (or higher) IM+IN dose to get a huge spike (100 ng/mL+) cMAX in tissue levels, most especially blood brain barrier penetration, huge mTORC1 inhibition pulse, but then letting sirolimus washout before next dose, to reduce mTORC2 inhibition, and immunosuppression before next dose. I will let the labs dictate, but for example, 4 weeks before next dose.
Regarding rapamycin and BBB penetration. Very clearly, one needs a relatively very high dose to penetrate.
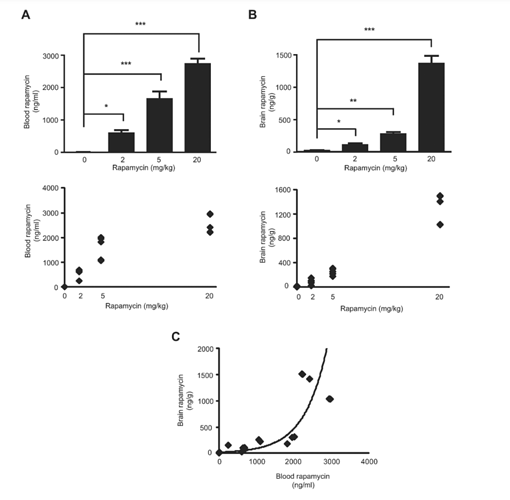
Interpreting Mammalian Target of Rapamycin and Cell Growth Inhibition in a Genetically-Engineered Mouse Model of Nf1-Deficient Astrocytes (2011)
“Rapamycin was administered at the indicated doses (2 mg/kg, 5 mg/kg, or 20mg/kg) by daily intraperitoneal (i.p) injections of rapamycin dissolved in ethanol (5 days per week for 2 weeks total). Brain rapamycin levels are exponentially correlated with blood rapamycin levels. One of the major obstacles in translating preclinical drug studies to human clinical trials is a relative paucity of reported blood and target tissue drug levels. These observations demonstrated that intraperitoneal rapamycin administration increases both blood and brain levels. However, brain rapamycin levels were exponentially correlated with blood rapamycin levels”
“Phospho-S6 is not a reliable biomarker of rapamycin inhibition in the brain. No significant phospho-S6 reduction was observed at the 2mg/kg/day rapamycin dose. Similar to S6 activation, 4EBP1 phosphorylation was unchanged at the 2mg/kg/day dose, whereas significant reductions were observed both at 5 and 20mg/kg/day rapamycin doses. Collectively, these data demonstrate that inhibition of mTOR downstream signaling in the brain requires AT LEAST 5mg/kg/day rapamycin treatment”. To determine whether AKT activation was seen in the brain following rapamycin treatment, we measured AKT Ser473 phosphorylation in vivo, and found a 4-fold increase in AKT activation at 5mg/kg/day, but not at 2 or 20mg/kg/day. These results suggest that AKT activation might counteract the growth suppressive effects of rapamycin mediated mTOR inhibition. Collectively, these results suggest that effective brain glial cell growth inhibition by rapamycin REQUIRES DOSES that inhibit BOTH TORC1 (S6; 4EBP1, and STAT3) and TORC2 (AKT) downstream pathways.”
And this paper, which delivered rapamycin levels in the brain, showed cognitive improvements in wild type mice.
Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice (2012)
“Mice were fed chow containing microencapsulated rapamycin in the chow resulting in an average dose of 2.24 mg rapamycin per kg body weight/day (MAC: HED 13.7mg PER DAY equivalent 75kg human, ergo therapeutic dosing). C57BL/6J mice fed a diet containing 14 ppm encapsulated rapamycin had rapamycin brain concentrations of 8.65 ± 0.66 ng/mg wet weight, similar to the rapamycin levels found in plasma in the same animals. Adequate plasticity requires that TOR activity be within a range that allows for appropriate regulation of protein synthesis at synaptic sites. Our results suggest that an approximate 30% reduction in TOR activity in brain for 16 weeks or longer improves performance of C57BL/6 mice in tasks that involve long-term plasticity and are dependent on hippocampus or on hippocampus and prefrontal cortex. It is therefore conceivable that brain mTOR activity levels that are adequate during the reproductive years may become detrimental as mammals age (Blagosklonny, 2010). In agreement with this hypothesis, we and others previously showed that chronic (> 16 weeks) inhibition of mTOR activity by rapamycin in brain preserves cognitive function in mice modeling AD, possibly by increasing autophagy. In summary, the results of the present study demonstrate that chronic feeding with encapsulated rapamycin enhances memory in young C57BL/6 mice and delays cognitive decline associated with aging. Moreover, our results show that long-term feeding with encapsulated rapamycin has concomitant anxiolytic and antidepressant-like effects. Our findings are consistent with prior studies showing that long-term rapamycin treatment rescued learning and memory in mice modelling AD”
I see. The problem is one would need massive systemic dosing to deliver very high LOCAL eye tissue sirolimus levels; the key amended method protocol was to further localize the drug to the target tissue.
“While we found that repeated subconjunctival sirolimus administrations were well tolerated, a positive anatomic or functional effect on GA enlargement was not detected. As this may be due to insufficient drug delivery to the retina, the current study investigated sirolimus delivered via intravitreal injection, which can increase retinal drug concentrations.”
And there’s likely no way they are putting this patient population on very high immunosuppression like doses for a local eye disease; superiority of outside the box IM+IN notwithstanding? The authors call out the fact the drug delivered to the eye did not impact systemically (by design). Trade a relatively lesser disease for a potentially avalanche of others. No ethics review board would likely allow, so likely reserved for us hackers! I’d rather inject my thigh than my eye, btw. ![]()
True - they are the guys who developed eRapa for the mouse studies… and they are trying to make a little money off it. Given that they contributed so much to the rapamycin longevity field, I don’t have a problem if they make a little money from it. I really can’t see them making much money though… I mean how many house and monkey trials will there be? I really don’t see much of a market for the product beyond animal testing. It seems they may be trying to expand to human applications - but they can’t really compete against the Indian generic companies… so perhaps they will move towards injectables. But these guys are scientists - not biotech guys.
Ah - just saw this
Exactly, some significant human clinical trial work/planned. EMTORA is promoting eRAPA like any other pharma ($$$) corporation. Appears they did finally get a patent issued in 2021
https://image-ppubs.uspto.gov/dirsearch-public/print/downloadPdf/11110067
eRAPA is simply a micro-encapsulation to improve bioavailability of rapamycin using a 3rd party technology (Eudragit100)
EUDRAGIT® Functional Polymers for Oral Solid Dosage Forms - Evonik Industries)
Benefits of eRapa™ over rapamycin and current rapalogues include:
- Enhanced bioavailability improves drug absorption, allows for a lower therapeutically effective dose, and improves its toxicity profile. eRapa™ consistently provides approximately 30% more drug than generic rapamycin (Emtora Biosciences unpublished data);
- Less interpatient pharmacokinetic variation reduces need for therapeutic drug monitoring and limits adverse events;
- Targeted delivery to the duodenum, lower intestine and colon delivers the active ingredient directly to the site of active disease in GI indications. eRapa has a systemic effect and a local (GI) effect.
Their original IP motivation (and still is) was to somehow leverage the original mice/rapamycin longevity work via a higher oral bioavailability delivery agent composition vs. raw rapamycin. They noted in their original mouse chow preparations (patent disclosure), they were able to use LESS rapamycin (thus much lower lab trial costs) by micro-encapsulation. There is nothing in the patent disclosures that supports current marketing hyperbole re “superior” therapeutic efficacy other than higher oral bioavailability. Oral still suffers from massive 1st pass metabolism effects, already thoroughly discussed.
It’s not clear to me from reading the patents and disclosures, that just taking standard and cheaper (even factoring perhaps higher dosing to compensate for the 30% less bioavailability) Rapamune/generics won’t accomplish similar.
It’s also quite interesting their disclosed mechanism of action:
"Mechanism of Action
eRapa™ modulates the mTOR pathway, which controls cell growth, proliferation, nutrient transport, autophagy, and survival. Chronic, intermittent, low dose eRapa™:
- Rejuvenates the immune system;
- Suppresses cancer in cancer-prone animals and prolongs health span and life span
- Enhances immature and naïve populations of lymphocytes;
- Decreases populations of accumulated (age) or induced (cancer) PD-1 expressing (exhausted) T cells."
A not so subtle and HUGELY different HUMAN translation dosing protocol vs the mice longevity work??
In US patent: https://image-ppubs.uspto.gov/dirsearch-public/print/downloadPdf/11110067
It is disclosed as matter of rapamycin mice longevity example “the encapsulated rapamycin was administered at 2.24 mg/kg PER DAY. The average blood level after feeding encapsulated rapamycin was GREATER than 25 ng/mL”. The HED would be about 14 mg PER DAY for an equivalent 75kg human, not factoring the higher bioavailability of eRAPA.
So how is this “intermittent, low dose” per their marketing MOA?
Why the different language for human translation? This is completely inconsistent with the patent mice longevity examples disclosure?
They DO disclose the concept of activation of AKT Ser463 with chornic treatment as a possible negative outcome feedback loop, a WELL KNOWN effect of chronic mTOR inhibitor use. This can actually cause cancer proliferation!
But they DISMISS this as an issue with chronic long term high dose rapamycin use?
https://image-ppubs.uspto.gov/dirsearch-public/print/downloadPdf/11110067
"For clinical applications, a major concern is that chronic application of rapamycin or rapalogs in a cancer prevention protocol may result in an increase in Akt Ser463 phosphorylation, which, as a PRO GROWTH stimulus (reviewed in Guertin and Sabatini 2009; Lane and Breuleux, 2009), would counteract any repressive effect. Recent immunoblot data from our lab indicates that this does not happen in normal fat and skeletal muscle in a long-term treatment setting. To illustrate, FIG. 32 shows immunoblot assays of visceral fat dissected from mice consuming food with rapamycin for 5 weeks. There was a significant induction in Akt Ser473 phosphorylation in response to this relatively short treatment. In contrast, visceral fat from mice treated with rapamycin for 20 months does not show this activation, and in males is significantly reduced (FIG. 33). The same trend is seen in skeletal muscle (FIG. 34). These data suggest that chronic treatment with enterically delivered rapamycin does not enhance tumor promoting activation of Akt, in somatic tissues but rather may reduce it."
So again, why the difference in human clinical translation language??
Clearly, profoundly, and humbling…MICE ARE NOT HUMANS.
Just did 5mg rectally. ![]()
The solubility in water is poor, so you have to draw it up as suspension a few times to get most of it. With another solvent might be interesting, as I guess it’s possible you could inhibit mTOR in lower GI tract+skip first-pass/not overdo mTOR inhibition in liver.
Probably will try intranasal next week.
So you used a water based suspension - nothing to dissolve at all? Yes, the solubility in water is poor, I’m not sure if any absorption in rectum could be expected like this?
I’ve used water-based suspensions for some obscure tryptamine and arycyclohexylamine salts in the past, and the bioavailability didn’t seem to be impacted. Ofc rapa is a larger molecule that doesn’t have overt psychoactive effects, so it’s harder to say whether it was actually absorbed.
Title:
Subcutaneous Rapamycin Injection Protocol — 10 mg Weekly Dose, 0.5 ml Volume, DMSO/PEG-400/PBS Vehicle (No Oil)
Hello everyone,
I wanted to share a detailed and practical protocol for subcutaneous rapamycin injections that I have developed, based on discussions here and available literature. The goal was to create a sterile, convenient, and bioavailable preparation without the use of oil vehicles.
Protocol summary:
- Dose: 10 mg rapamycin per injection
- Frequency: once weekly
- Injection volume: 0.5 ml per dose (small volume for comfort)
- Vehicle composition: 20% DMSO, 40% PEG-400, 40% sterile PBS (pH 7.4)
- Total preparation: 100 mg rapamycin dissolved in 5 ml vehicle, divided into 10 × 0.5 ml sterile syringes
- Storage: syringes frozen at -20°C, thawed immediately before use, no refreezing
- Administration: subcutaneous injection (abdomen or thigh), injection done slowly to minimize discomfort
Preparation steps:
- In a sterile environment, dissolve 100 mg rapamycin in 1 ml DMSO until fully dissolved.
- Add 2 ml PEG-400 and mix thoroughly until clear.
- Add 2 ml sterile PBS (pH 7.4) and mix gently.
- Using a 0.22 µm sterile syringe filter, filter the solution directly into 10 sterile insulin syringes (0.5 ml per syringe).
- Cap syringes with sterile caps or needle covers and freeze immediately at -20°C.
Notes:
- This vehicle composition balances solubility and tissue tolerance, avoiding the complexity and sterility challenges of oil-based depots.
- The protocol aims for practical weekly dosing with acceptable bioavailability and minimal irritation.
- The volume of 0.5 ml per injection improves patient comfort for subcutaneous delivery.
- Freezing aliquots individually maintains sterility and stability over 10 weeks.
- Slow injection technique recommended.
If anyone has experience with alternative vehicles, depot methods, or different dosing strategies, I’d love to hear your feedback.
Thanks for reading!