Ordered some from India.
For me it’s more a replacement for Plioglitazone which works well but has some safety issues.
Also my DEXA fat % went from 14% to 16% with Plioglitazone.
Metformin did not do anything at all for me.
I did a Gemini Pro deep research: Bioenergetic Pharmacotherapy in Type 2 Diabetes: A Comprehensive Analysis of Imeglimin Interactions, Comparative Safety, and Physiological Adaptations
That includes interaction with rapamycin, empagliflozin, exercise, etc.
Table 1: Comparative Safety and Efficacy Profile: Imeglimin vs. Pioglitazone
| Feature | Pioglitazone (TZD) | Imeglimin (Glimin) | Clinical Consequence |
|---|---|---|---|
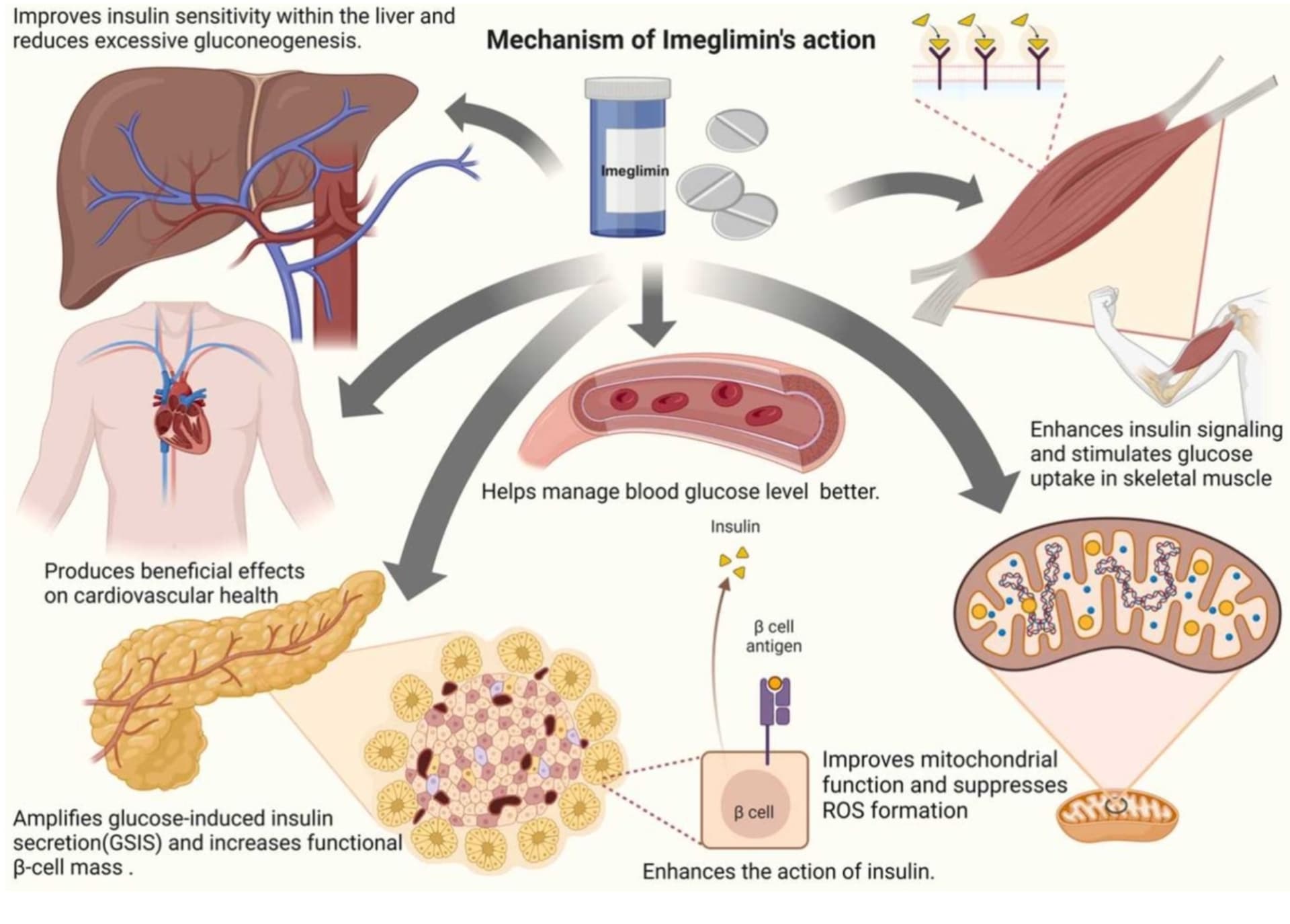
| Primary Target | Nuclear PPARγ Receptor | Mitochondrial Complex I/III | Imeglimin avoids nuclear transcription side effects. |
| Heart Failure Risk | Increased (Boxed Warning) | Neutral (Potential Benefit) | Imeglimin is safer for patients with cardiac history. |
| Edema | Frequent (Renal Na+ reabsorption) | Rare / Absent | Imeglimin preferred in patients prone to volume overload. |
| Bone Fracture Risk | Increased (Marrow adipogenesis) | Neutral | Imeglimin preferred in postmenopausal women/elderly. |
| Weight Effect | Weight Gain (Adipogenesis + Fluid) | Neutral | Imeglimin avoids exacerbating obesity. |
| Liver Fat | Reduces (Potent) | Reduces (Moderate) | Both benefit MASH, but Pioglitazone has more data. |
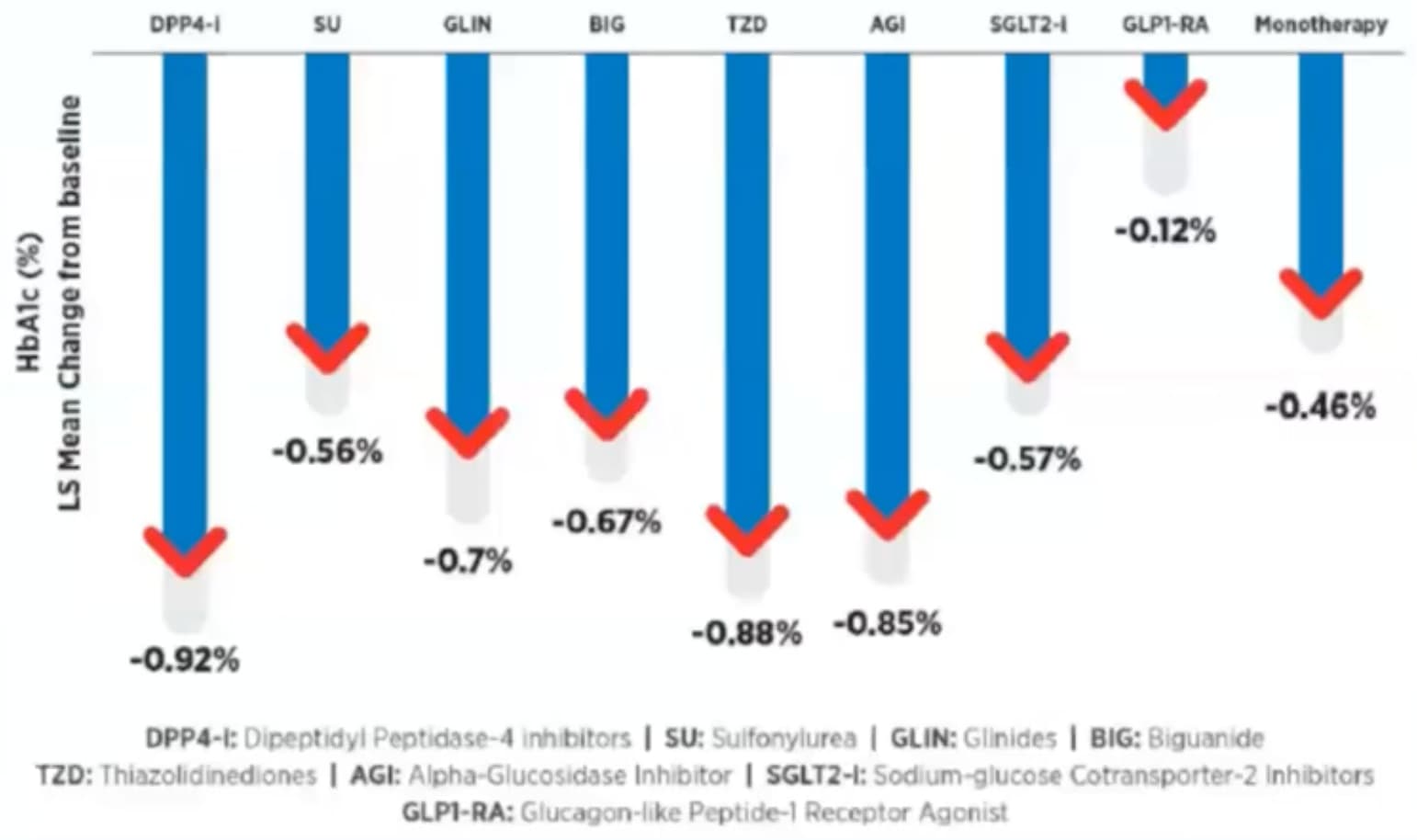
| Onset of Action | Slow (Weeks/Months) | Rapid | Imeglimin offers faster glycemic control. |
Table 2: Mechanism of Interaction: Imeglimin + Empagliflozin
| Component | Empagliflozin Contribution | Imeglimin Contribution | Synergistic Outcome |
|---|---|---|---|
| Glycemia | Urinary glucose excretion (Insulin Independent) | Insulin sensitization + GSIS (Insulin Dependent) | Potent HbA1c reduction covering all mechanisms. |
| Cardiac | Hemodynamic unloading + Ketone fuel | Mitochondrial efficiency + Endothelial function | Structural and metabolic cardiac remodeling. |
| Renal | Reduced glomerular pressure | Reduced oxidative stress | Preservation of nephron mass. |
| Liver | Reduced hepatic fat | Reduced lipogenesis + fibrosis | Mitigation of MASH progression. |
Table 3: Summary of Imeglimin Interactions and Effects
| Domain | Interaction / Comparison | Key Outcome / Mechanism |
|---|---|---|
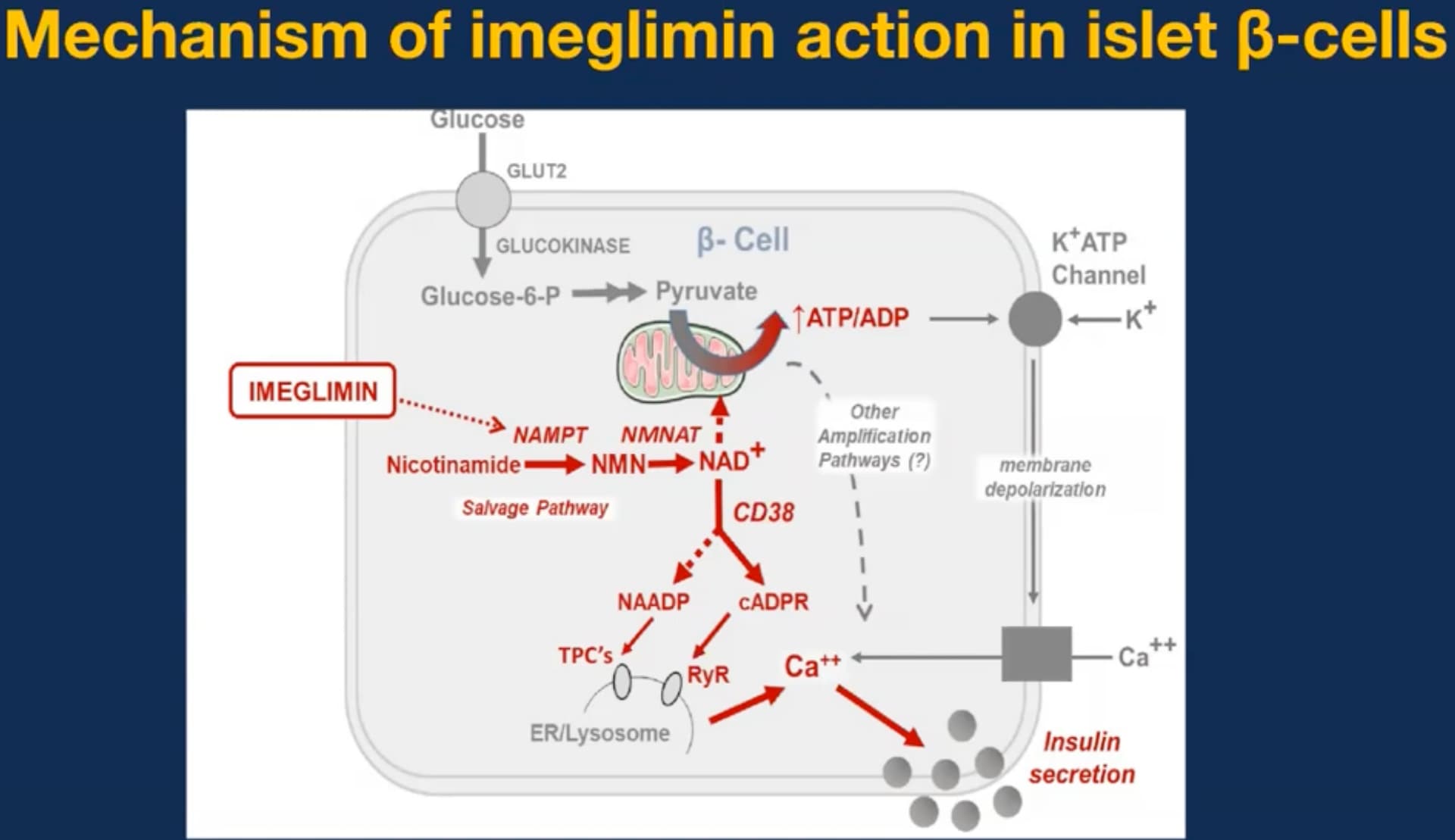
| Pharmacology | vs. Metformin | Competitive vs. Non-competitive Complex I inhibition; Imeglimin rescues Complex III and amplifies GSIS. |
| Safety | vs. Pioglitazone | Imeglimin has NO heart failure, edema, or bone fracture risk. Weight neutral. |
| Synergy | w/ Tirzepatide | Complementary beta-cell effects: Tirzepatide stimulates, Imeglimin protects (mitochondrial buffering). |
| Synergy | w/ Empagliflozin | Full Cardio-Renal protection: SGLT2i (hemodynamic) + Imeglimin (metabolic/endothelial). |
| Longevity | w/ Rapamycin | Imeglimin (AMPK/SIRT1) rescues mitochondrial biogenesis inhibited by Rapamycin (mTORC1 blockade). |
| Muscle | Strength Training | Increases strength (~13%) without hypertrophy. Improves NMJ/Bioenergetics. |
| Muscle | Endurance | Enhances PGC-1α and mtDNA. Potentially additive to exercise adaptations. |
| Renal | TWINKLE Study | Safe in eGFR < 45 mL/min (unlike Metformin). No lactic acidosis signal. |