Is there any studies on any drug that inhibit IGF-1 and which has shown good lifespan extension?
IGF1 receptor inhibition amplifies the effects of cancer drugs by autophagy and immune-dependent mechanisms (2021)
“Here, we report the identification of picropodophyllin (PPP), a cyclolignan alkaloid derived from the mayapple plant, as a potent inducer of autophagy that acts through the inhibition of insulin-like growth factor-1 receptor (IGF1R). When administered to tumor-bearing mice, PPP enhanced the
efficacy of immunogenic chemotherapy combined with immunotherapy and these effects relied on the induction of autophagy in malignant cells… PPP efficiently blocked IGF1-induced IGF1R autophosphorylation, the activating phosphorylation of protein kinase B (best known as AKT), the phosphorylation of MTOR, and the activity of MTOR complex 1 (MTORC1), evaluated by assessing the phosphorylation of the MTORC1 substrates p70S6K and TFEB. Inhibition of IGF1R itself or that of the signal transduction cascade acting downstream of IGF1R (the PI3K/AKT/MTOR pathway) potently stimulates autophagy as well as other stress-adaptive mechanisms. Indeed, a vast literature suggests that chronic inhibition of this pathway by caloric restriction pharmacological inhibitors or loss-of-function mutations has the capacity to extend the healthspan and lifespan in model organisms and perhaps in humans as well. PPP could be replaced by another IGF1R inhibitor, linsitinib, which has undergone evaluation in clinical trials”
IGF-1R Inhibitor Ameliorates Neuroinflammation in an Alzheimer’s Disease Transgenic Mouse Model (2020)
Clinical setbacks reduce IGF-1 inhibitors to cocktail mixers (2012, existing cancer trial)
https://doi.org/10.1038/nbt1012-906c
“AMG-479’s failure will likely be a death knell for the field of IGF-1 targeting. For the meantime,
though, it looks like industry will be looking at mechanisms other than IGF-1R inhibition
in cancer drug development”
Enclomiphene and clomiphene lower IGF in general and when used as TRT. Apparently exogenous T does not.
To actually answer your question ![]() : only one “drug” that blocks IGF-1 signaling has been tried, and the results were not exactly what you would want:
: only one “drug” that blocks IGF-1 signaling has been tried, and the results were not exactly what you would want:
Blockquote
To this end, we performed a preclinical study in 18-mo-old male and female mice treated with vehicle or an IGF-1R mAb (L2-Cmu, Amgen Inc), and determined effects on aging outcomes. Here we show that L2-Cmu preferentially improves female healthspan and increases median lifespan by 9% (P = 0.03) in females, along with a reduction in neoplasms and inflammation (P ≤ 0.05). … However, despite improved survival and less cancers with mAb treatment, no significant effect was observed on maximum lifespan (P = 0.971). …
[Long-term L2-Cmu treatment tended to reduce female body weight and lean body mass, with no effect on adiposity. It is unclear to what extent the reduction in lean mass is attributable to loss of skeletal muscle mass per se, which comprises only one component of total body lean mass in mice
male interim survival with L2-Cmu was indistinguishable from Controls … tumor burden in males tended to be increased …
Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice | Nature Communications
While insulin-like growth factor-1 (IGF-1) is a well-established modulator of aging and longevity in model organisms, its role in humans has been controversial. In this study, we used the UK Biobank (n = 440,185) to resolve previous ambiguities in the relationship between serum IGF-1 levels and clinical disease. We examined prospective associations of serum IGF-1 with mortality, dementia, vascular disease, diabetes, osteoporosis, and cancer, finding two generalized patterns: First, IGF-1 interacts with age to modify risk in a manner consistent with antagonistic pleiotropy; younger individuals with high IGF-1 are protected from disease, while older individuals with high IGF-1 are at increased risk for incident disease or death. Second, the association between IGF-1 and risk is generally U-shaped, indicating that both high and low levels of IGF-1 may be detrimental. With the exception of a more uniformly positive relationship between IGF-1 and cancer, these effects were remarkably consistent across a wide range of conditions, providing evidence for a unifying pathway that determines risk for most age-associated diseases. These data suggest that IGF-1 signaling could be harmful in older adults, who may actually benefit from the attenuation of biological growth pathways.
Full Open Access Paper: The antagonistic pleiotropy of insulin‐like growth factor 1 - PMC
Just as an aside…
The company Loyal for Dogs is coming out with a new IGF-1 inhibition drug to extend life in dogs (and eventually humans). But there is already an IGF-1 inhibition drug approved for people.
I came across this paper:
see this section:
Inhibitors of the GH/IGF-1 axis
Although the lack of global IGF-1 signaling is lethal, data from studies conducted in animal models have shown that a reduction in IGF-1 levels or IGF-1 action can extend lifespan. Additionally, human IGF-1 receptor gene polymorphisms are associated with exceptional longevity (Suh et al., 2008), and recently, Barzilai and colleagues have shown that low plasma IGF-1 concentrations predict survival in long-lived people (Milman et al., 2014), specifically in women with a history of cancer. In animals, dwarf, long-lived mice lacking the growth hormone receptor (GHR-/-) have reduced levels of IGF-1, are insulin sensitive despite obesity, and have decreased risk for cancer and diabetes (Zhou et al., 1997; Shevah & Laron, 2007; Ikeno et al., 2009). Importantly, similar results have been reported in growth hormone receptor-deficient Laron syndrome (LS) patients. In this regard, no formal aging studies have been performed on patients with LS; however, they are protected from diabetes and fatal neoplasms. (Guevara-Aguirre et al., 2011; Steuerman et al., 2011). Thus, pharmaceutical interventions that directly lower IGF-1 levels in adults could improve health and prolong lifespan.
Pharmacological targets for lowering IGF-1 action include those that act directly or indirectly on cells/tissues that produce or respond to GH and/or IGF-1. In this regard, human or humanized monoclonal antibodies and drugs directed against the IGF-1R have been used in clinical trials to treat several types of cancer (Warshamana-Greene et al., 2005; Carboni et al., 2009); however, none have been approved for clinical use. We are unaware of the development of any antibody against GH or IGF-1, but several classes of compounds that inhibit the GH/IGF-1 axis have been approved for use in patients with acromegaly. Recently, a consensus document has been developed for the use of this therapeutics (Giustina et al., 2014). One of these drug classes, somatostatin analogues, lower serum GH levels by suppressing GH secretion by pituitary somatotrophs, thereby ultimately decreasing serum IGF-1 levels. Unfortunately, these compounds also suppress secretion of other endocrine hormones, including insulin. Furthermore, only 20-50% of patients with acromegaly respond to these drugs, and significant adverse events have been documented including gallstones, diarrhea, and anorexia. Thus, the use of somatostatin analogues to increase longevity or healthspan appears to be unwarranted at this time.
The second approved drug for treating acromegaly is the GH receptor antagonist pegvisomant (Trainer et al., 2000; van der Lely et al., 2001; Kopchick et al., 2002; van der Lely & Kopchick, 2006). Pegvisomant is unique in that it does not inhibit GH secretion, but rather inhibits GH action by binding to and blocking the GHR (Kopchick et al., 2002). Notably, a dose-dependent decrease of IGF-1 levels is seen in up to 90% of pegvisomant-treated patients (Trainer et al., 2000; van der Lely et al., 2001; Kopchick et al., 2002; van der Lely & Kopchick, 2006). Additionally, pegvisomant is an insulin sensitizer that blocks the diabetogenic action of GH and thus produces beneficial effects on glucose metabolism. Pegvisomant, therefore, could have positive effects on both longevity and healthy aging by lowering serum IGF-1 and increasing insulin sensitivity. Regarding adverse effects, van der Lely et al. (2012)reported that Long-term data on the efficacy and safety profile of pegvisomant are reassuring and few long-term serious adverse events have been reported but ongoing vigilance is required to monitor liver function and tumor size. Thus, pegvisomant is an approved drug that should be tested for its effects on longevity and healthy aging. Future therapeutics targeted at inhibiting the GH/IGF-1 axis could include small inhibitory RNAs directed against the GHR or IGF-1 receptor mRNAs, monoclonal antibodies directed against GH or IGF-1, or novel GHR or IGF-1R tyrosine kinase inhibitors.
Another way to reduce global IGF-1 action may be to inhibit IGF-1 availability. For example, loss of PAPP-A, a protease that cleaves and inactivates the IGF-1 sequestering protein IGFBP-4, reduces IGF-1-induced signaling without affecting overall serum IGF-1 levels and not only extends mouse lifespan, but has many other beneficial effects on healthspan and age-related diseases (Conover, 2012).
In summary, reducing the activity of the GH/IGF-I somatotrophic axis is perhaps the most validated and consistent genetic intervention to extend mouse lifespan and healthspan. In addition GHR/IGF-I deficiency is also among the few phenotypes that is well characterized in humans (patients with Laron syndrome) with very few side effects in adults, even considering the extreme level of GH receptor deficiency and the resulting >80% reduction in circulating IGF-I. Notably, a pharmaceutical intervention targeting this pathway may or may not be designed to achieve such a low level of hepatic IGF-I secretion
and this paper:
its extremely likely that this drug called somavert extends lifespan in mammals…
Injection site common side effect…
https://www.pfizermedicalinformation.com/en-us/node/62776/pi_section/field_spl_instructions
“Pegvisomant is unique in that it does not inhibit GH secretion, but rather inhibits GH action by binding to and blocking the GHR. pegvisomant is an insulin sensitizer that blocks the diabetogenic action of GH and thus produces beneficial effects on glucose metabolism. Pegvisomant, therefore, could have positive effects on both longevity and healthy aging by lowering serum IGF-1 and increasing insulin sensitivity”
“Low” protein diet works.
“Reducing protein intake from an average of 1.67 g kg−1 of body weight per day to 0.95 g kg−1 of body weight per day for 3 weeks in six volunteers practicing CR resulted in a reduction in serum IGF-1 from 194 ng mL−1 to 152 ng mL−1…In addition, our data provide evidence that protein intake is a key determinant of circulating IGF-1 levels in humans, and suggest that reduced protein intake may become an important component of anticancer and anti-aging dietary interventions.”
These seem to be very important and under-appreciated data, hypotheses and conclusions?
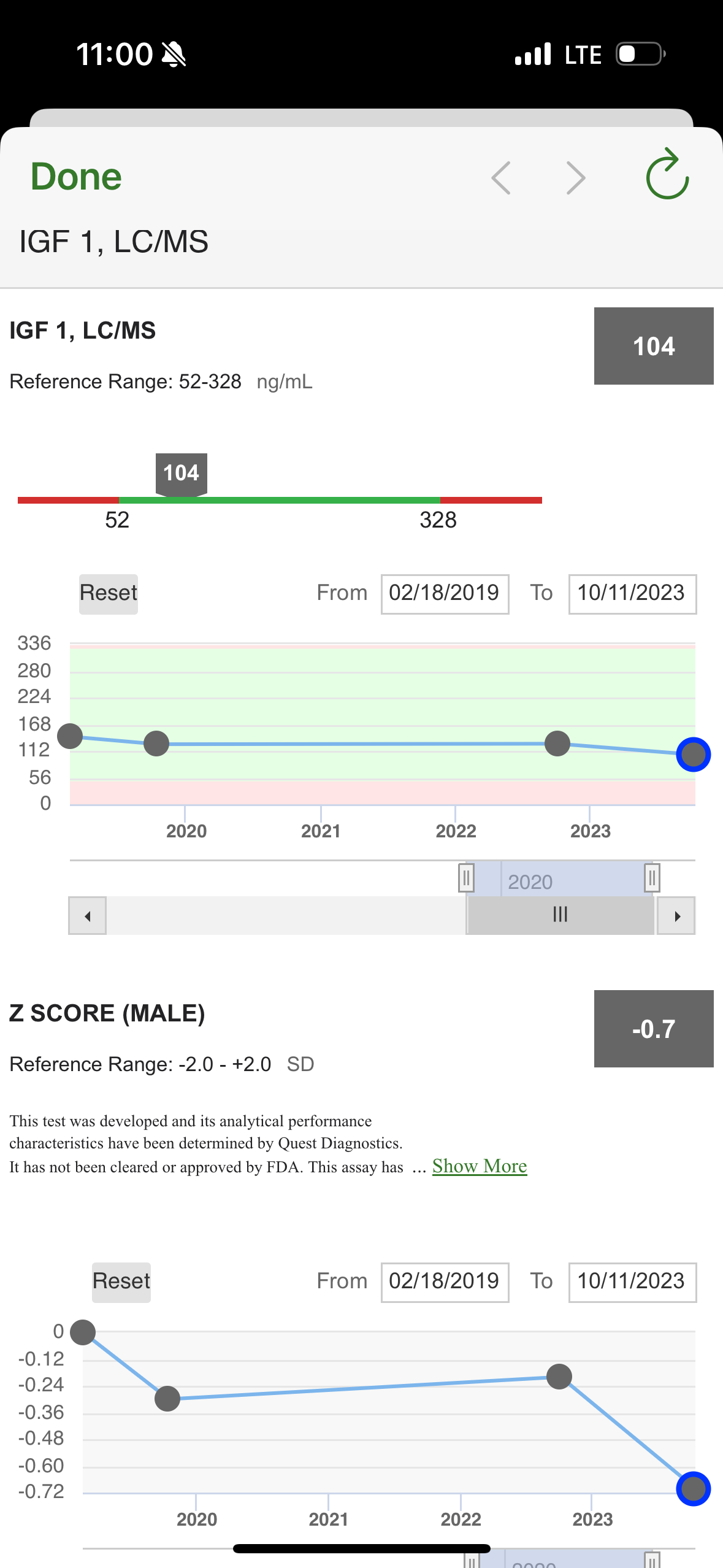
Does anyone have experience of testing IGF-1 levels? What provider/type of test do you prefer, any things one should take into account before starting a testing program for it? How infra day (or hour) variable is it?
I agree - something we need to track, and manage, and perhaps target to lower it at times.
I’ve had it tested, but don’t do it very often (its not one of my main biomarkers I track) - but perhaps it should be.
Rapamycin “should” lower IGF-1 according to the research, but I wonder by how much / how dose-responsive it is…
We can get it cheaply tested - see here from Mareck for $39
LifeExtension Foundation is $75
But I’ve done no research on what the target optimal range might be (for all cause lowest mortality?).
Good questions, you ask, but I don’t have an answer yet. Lets dig into this one!
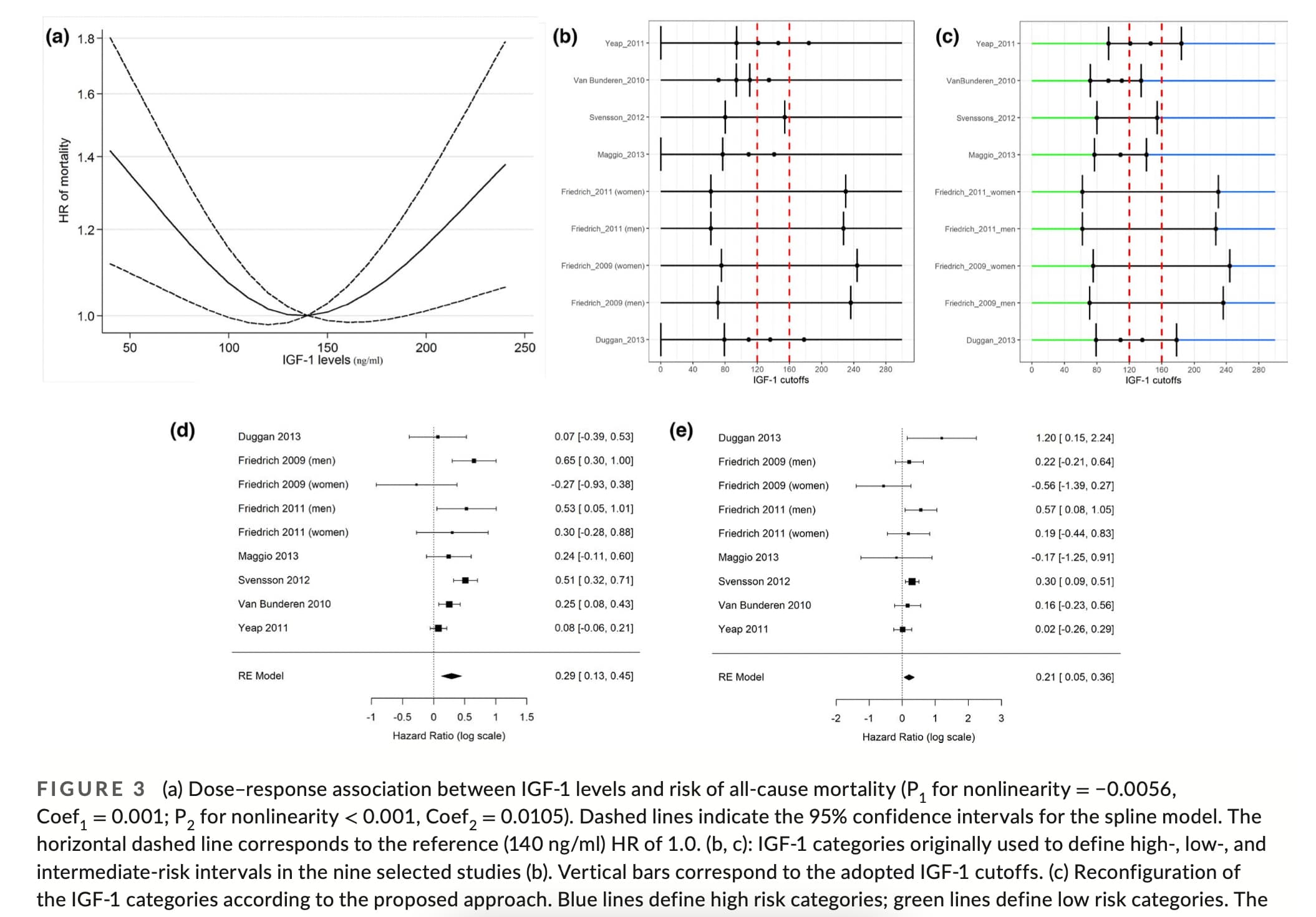
It seems the data is pretty messy… but a target range on the lower end of this range seems reasonable: “the low‐ to mid‐range levels of IGF‐1 (120–160 ng/ml) are reflective of the “healthiest” status.” (but Laron Syndrome people have almost zero IGF-1 and have many health benefits).
Association between IGF‐1 levels ranges and all‐cause mortality: A meta‐analysis
A series of studies have shown that high levels of IGF‐1 are associated with an increased risk of tumors including prostate, pre‐ and postmenopausal breast, lung, thyroid, and colorectal cancers (Ma et al., 1999; Renehan et al., 2004; Shi et al., 2001). An increase in serum IGF‐1 level of 100 ng/ml was shown to correspond to a 69% increase in colorectal cancer risk (Ma et al., 1999). High levels of IGF‐1 were also shown to be associated with a 49% increase in prostate cancer, 65% increase in breast cancer (Renehan et al., 2004), and a 106% increase in lung cancer risk (Yu et al., 1999). Furthermore, in worms, flies, and mice insulin/IGF‐1 signaling reduces lifespan and healthspan (Bartke et al., 2013; Fontana et al., 2010; Kenyon, 2010; Podshivalova et al., 2017).
…
In mice, very low levels of IGF‐1 are associated with reduction in a range of diseases and conditions including cancer, diabetes, and cognitive decline and with record longevity (Bartke et al., 2013). In fact, mice with severe IGF‐1 deficiency, achieved by either growth hormone receptor (GHRD) or GH deficiency, display a 40% extended longevity (Bartke et al., 2013). Also, GHRD mice are protected from age‐related decline in memory and perform similarly to young normal mice. Additionally, insulin/IGF‐signaling (IIS) pathway accelerates aging in Caenorhabditis elegans and the fly D. melanogaster (Bartke et al., 2013).
In humans, studies on patients with Laron syndrome (LS), whose IGF‐1 levels are extremely low, have reported a reduction in pro‐aging signaling, cancer, diabetes, and cognitive decline (Guevara‐Aguirre et al. 2011; Nashiro et al. 2017). Steuerman et al. (2011) also surveyed 230 individuals with LS and found no cases of cancer.
On the contrary, other studies reported an association between low levels of IGF‐1 and conditions like CVD, diabetes mellitus, osteoporosis, and sarcopenia although a causal relationship has not been established (Brioche et al., 2014; Katsanos et al., 2001; Lenk et al., 2010; Saki et al., 2017).
In summary, because extensive data in both mice and humans consistently show that even extremely low levels of IGF‐1 are associated with increased lifespan or healthspan and in agreement with the meta‐analysis presented here, we propose that low‐ to mid‐range levels of IGF‐1 (120–160 ng/ml) are reflective of the “healthiest” status.
Source:
Interestingly… in this study of Laron syndrome (Growth Hormone Receptor Deficiency/GHRD):
the “Serum IGF-I ranged from 29 to 310 ng/ml (mean 144) among relatives, but was ≤ 20 ng/ml in all GHRD subjects”
These people don’t get cancer, and are very low on the scale for other age related disease: diabetes, and cognitive decline.
(Guevara‐Aguirre et al. 2011; Nashiro et al. 2017). Steuerman et al. (2011) also surveyed 230 individuals with LS and found no cases of cancer.
It would seem that cycling IGF-1 levels down to near zero for significant periods of time tends to be helpful to mitigate age related diseases…
The half-life for Pegvisomant is about 75 hours… so dosing strategy might be similar to rapamycin, of weekly. Pegvisomant for acromegaly - PMC
But, rapamycin also inhibits IGF-1 - so perhaps for rapamycin users there is no need for additional IGF-1 inhibition…
Methionine restriction (MR) dramatically extends the healthspan of several organisms. Methionine-restricted rodents have less age-related pathology and increased longevity as compared with controls, and recent studies suggest that humans might benefit similarly. Mechanistically, it is likely that the decreased IGF-1 signaling that results from MR underlies the benefits of this regimen. Thus, we hypothesized that interventions that decrease IGF-1 signaling would also produce MR-like healthspan benefits. Selenium supplementation inhibits IGF-1 signaling in rats and has been studied for its putative healthspan benefits. Indeed, we show that feeding mice a diet supplemented with sodium selenite results in an MR-like phenotype, marked by protection against diet-induced obesity, as well as altered plasma levels of IGF-1, FGF-21, adiponectin, and leptin. Selenomethionine supplementation results in a similar, albeit less robust response, and also extends budding yeast lifespan. Our results indicate that selenium supplementation is sufficient to produce MR-like healthspan benefits for yeast and mammals.
Low IGF-1 as a kid would be a bummer. But as an adult….
Perhaps this is one of those stuck-in- growth mode things that rapa and CR helps with.
Methionine activates MTOR and inactivates AMPK. It’s kind of the anti-Rapamycin. So it makes sense that reducing it would extend life expectancy.
Thanks @RapAdmin for of this valuable info, very helpful and valuable!
Has anyone measured and seen how IGF-1 changes with rapa N=1?
This really fits with things I’m trying to find and create strategies for - cancer and neurodegen as 2 of the bid 4 seems so much less under our control than cardiovascular and metabolic disease…
That’s what I’m thinking.
From LEF it seems that:
“IGF-1 appears constant over a 24-hour period, making it useful as a bio-marker to help assess blood levels of GH.”
And
”Fasting is not required for this test. Take all medications as prescribed.”
And from Marek it seems like it one the tests that (at least with some assays) can get wacko results if done while having somewhat recent vitamin B7 supplementation:
”Note: This test may exhibit interference when sample is collected from a person who is consuming a supplement with a high dose of biotin. Cease biotin supplementation at least 72 hours prior to blood draw.”