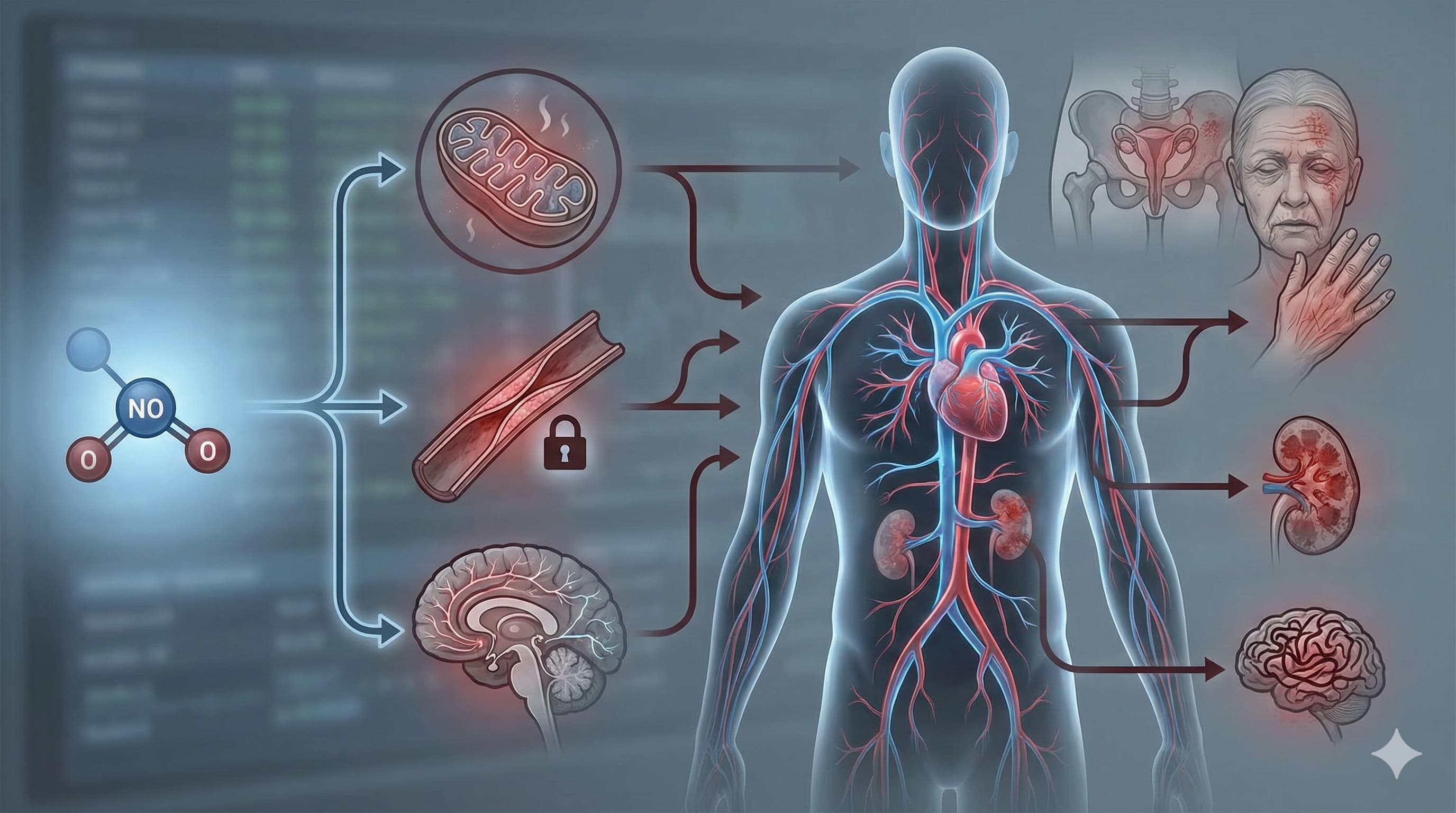
Nitric Oxide (NO) is frequently misunderstood as merely a vasodilator or a performance-enhancing supplement. However, this review establishes NO as a foundational “molecular currency” of aging that undergoes a catastrophic devaluation as we get older. The central thesis is that aging is not just a passive decline in NO levels, but an active dysregulation where the machinery meant to produce NO—specifically endothelial Nitric Oxide Synthase (eNOS)—breaks down (“uncouples”) and begins manufacturing damaging free radicals instead.
This physiological switch creates a vicious cycle: as NO bioavailability drops, mitochondrial function falters, vascular stiffness increases, and cognitive decline accelerates. The review painstakingly maps this failure across multiple organ systems, linking the loss of NO signaling to erectile dysfunction, skin aging, renal failure, and neurodegeneration. Crucially, the paper argues that restoring the “coupling” of NO synthase—rather than just blasting the system with arginine—is the key therapeutic target. It highlights a convergence of synthetic drugs (like PDE5 inhibitors) and natural compounds (like resveratrol and icariin) that can reverse this uncoupling, potentially restoring youthful signaling phenotypes.
Source:
- Open Access Paper: An Overview of NO Signaling Pathways in Aging
- Impact Evaluation: The impact score of this journal is approximately 4.9 (Journal Impact Factor), therefore this is a Low/Medium impact journal.
Can Boosting Nitric Oxide Rescue Aging Arteries and Exercise Capacity?
Aging is fundamentally a vascular and mitochondrial disease, heavily mediated by the progressive loss of nitric oxide (NO) bioavailability. NO is a ubiquitous gaseous signaling molecule essential for regulating skeletal muscle blood flow, mitochondrial ATP production, and overall endothelial health. While sedentary aging decimates endogenous NO production—leading to a steady 1% annual decline in VO2max after age 30 and severely impaired functional sympatholysis—lifelong aerobic exercise appears to preserve it. Older “Masters athletes” maintain NO levels comparable to healthy young adults, effectively insulating themselves against primary vascular aging and delaying physiological decline.
The central question is whether exogenous supplementation can reverse age-related NO deficits in the broader population.
The clinical data reveals a sharply split verdict. Interventions targeting the canonical L-arginine/NOS pathway (L-citrulline, L-arginine) or the alternative nitrate-nitrite-NO pathway (inorganic nitrate, beetroot juice) successfully elevate surrogate systemic NO biomarkers in older adults. However, the translation of these biochemical markers into measurable endurance or exercise performance improvements is highly inconsistent. Dietary nitrate shows ergogenic promise primarily in older adults suffering from distinct clinical pathologies, such as chronic obstructive pulmonary disease (COPD) or heart failure. Conversely, performance benefits for healthy or highly trained older cohorts remain largely unproven, with several trials showing null results. Furthermore, dietary antioxidants and (poly)phenols fail to demonstrate reliable efficacy for enhancing NO or performance in this demographic. Consequently, while NO manipulation remains biologically plausible for extending healthspan, current over-the-counter protocols lack the rigorous, healthy-cohort validation required to guarantee functional outcomes in aging individuals.
Source:
- Open Access Paper: Nitric oxide, aging and aerobic exercise: Sedentary individuals to Master’s athletes
- Institution: Newcastle University, Loughborough University, and University of Colorado Boulder.
- Country: United Kingdom and United States.
- Journal Name: Nitric Oxide. This is a Medium impact journal.
Related Reading: