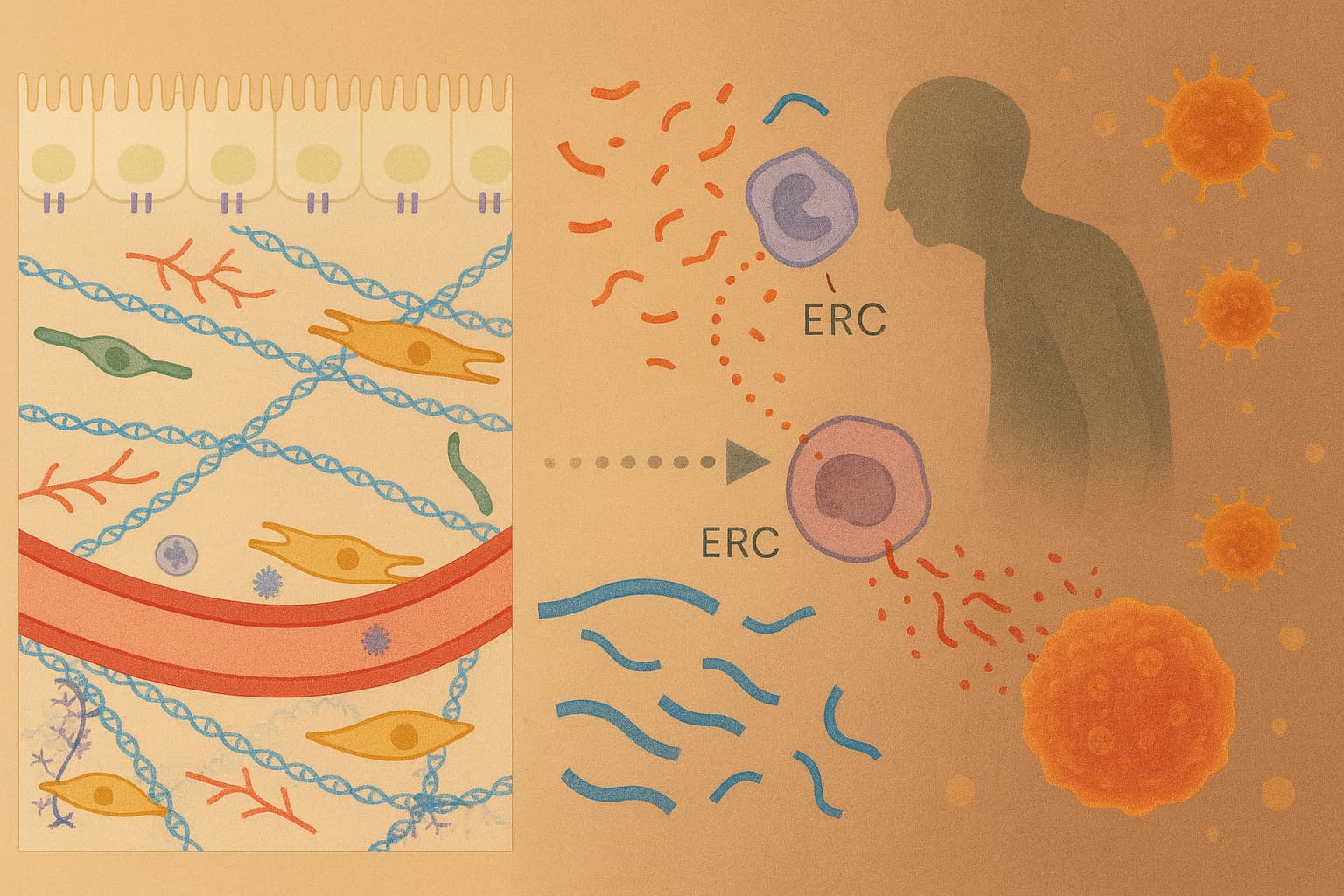
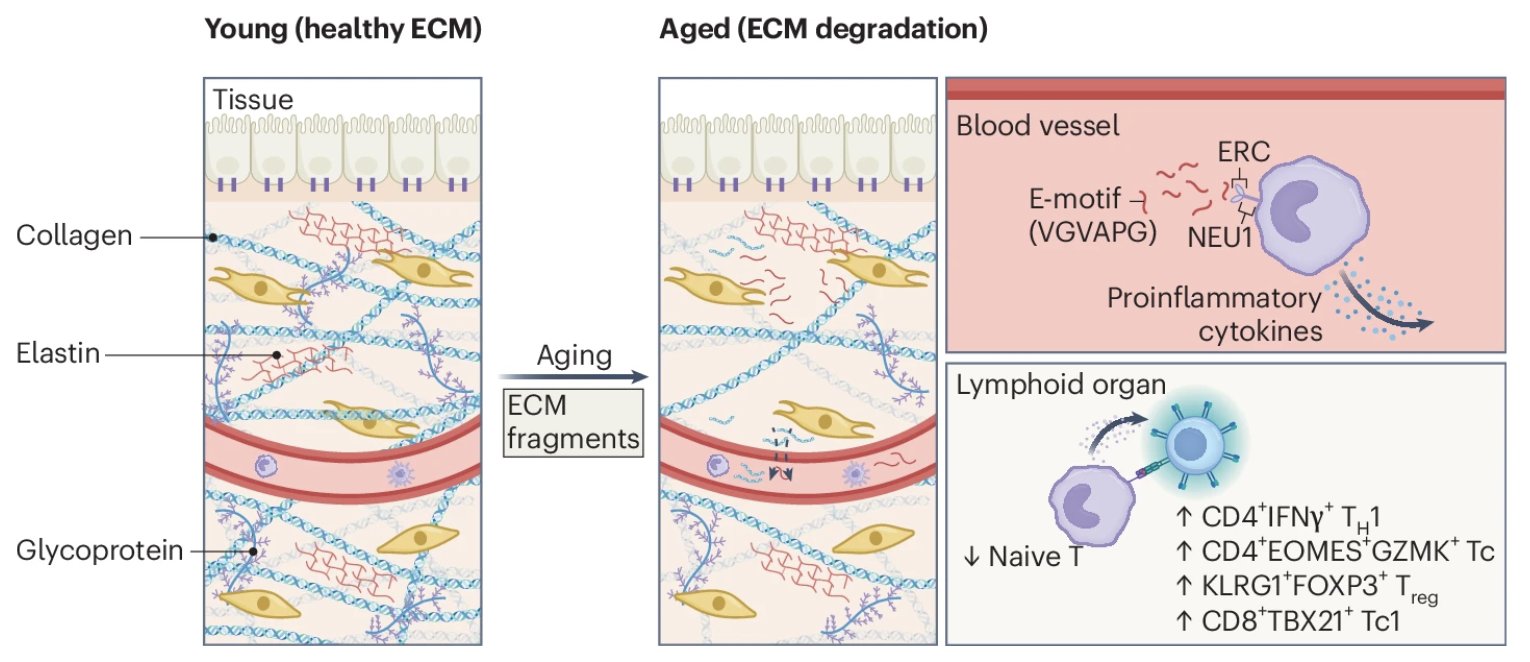
Some follow-on questions on the original research paper summarized:
I’ll go through the questions one by one and separate what’s known, what’s plausible, and what’s still guesswork. (Editor: I’ve eliminated some of the questions that were not very relevant here)
1. Can we measure elastin fragments (ELN fragments) in humans today?
Yes, but only as research assays, not standard clinical tests.
- Multiple companies sell human elastin-derived peptide (EDP) ELISA kits for serum/plasma (e.g., “Human EDP (Elastin-Derived Peptides) ELISA Kit” and similar). These are marketed strictly for research use, not for clinical decision-making.
- There are also ELISAs for total elastin in serum/plasma.
- In principle, targeted LC–MS/MS proteomics can quantify specific elastin peptides, and this is in line with what the Nature Aging paper did for murine/human cohorts (though their exact assays are not commercially packaged).
Gaps:
- No standardized reference ranges by age/sex.
- No CLIA-validated test you can order from a mainstream lab yet.
- No longitudinal human data on “track EDP every 6–12 months and correlate with outcomes.”
For a biohacker: feasible only via research collaboration or paying for custom proteomics / RUO ELISAs; not plug-and-play like hs-CRP.
2. Can lifestyle/environment reduce elastin fragmentation in humans?
We have strong indirect evidence; very limited direct EDP data.
Most of the following are almost certainly elastin-sparing, even if the exact EDP delta isn’t quantified:
- Smoking / inhaled toxins
- Smoking drives elastase-mediated destruction of lung elastin in COPD/emphysema and increases elastase in the circulation. Smoking cessation consistently slows radiographic and functional decline in lungs, which is mediated in part via lower elastase activity.
- Blood pressure and vascular stress
- Chronic hypertension accelerates arterial elastin fatigue and fragmentation, contributing to arterial stiffness and aneurysm formation. Tight BP control reduces progression of arterial stiffness and aortic dilation in multiple cohorts.
- UV exposure
- Photoaging literature is very clear that chronic UV causes solar elastosis and dermal elastin breakdown; UV-protected vs sun-exposed skin shows striking elastin degradation and abnormal elastin aggregates histologically.
-
Chronic lung disease control (COPD, bronchiectasis, severe asthma)
- These states are rich in neutrophil elastase. Neutrophil elastase inhibitors like sivelestat are used acutely in ARDS/ALI in Japan/China, precisely because NE drives lung injury.
- Oral and systemic inflammatory burden
- Periodontal disease, obesity, and metabolic syndrome all increase neutrophil and monocyte activation. They plausibly raise elastase activity and ECM turnover, though this is mostly extrapolated from inflammatory and MMP data not direct EDP measurements.
Take-home: if your goal is to minimize elastin fragmentation, the usual longevity hygiene (no smoking, low BP, low visceral fat, low chronic inflammatory load, UV protection, aggressive periodontal care) is highly coherent with the mechanism, even if we don’t yet have human EDP curves pre- and post-intervention.
4. Existing drugs that reduce elastase activity without insane risk?
Here we’re mainly talking about neutrophil elastase (NE) and related proteases .
4.1 Sivelestat
- A selective neutrophil elastase inhibitor used in Japan and parts of Asia for acute lung injury/ARDS, usually IV and short-term.
- Meta-analyses suggest modest benefit in ARDS; evidence in chronic respiratory disease (COPD, CF, bronchiectasis) is weak or negative.
- No long-term safety data for chronic use; no evidence for aging or ECM endpoints.
This is not a very practical longevity drug for most people. It’s approved only in Japan / Asia, and is a hospital-grade acute care drug with IV delivery. Hard to get, though cost is low because it seems to have gone generic (drug was introduced in 2002).
Grok: Sivelestat (also known as Elaspol) is not sold by any online pharmacies. It is a prescription medication approved only in Japan and South Korea.
Sivelestat (known as 西维来司他钠 or Elaspol in Chinese) is sold in China. It was approved by the National Medical Products Administration (NMPA) in March 2020 via an expedited emergency review process for treating acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) associated with systemic inflammatory response syndrome (SIRS), particularly during the COVID-19 pandemic. Developed by Shanghai Huilun Jiangsu Pharmaceutical Co., Ltd., it has been widely used in hospitals, with over 1.9 million units distributed by 2022 for ICU and respiratory care. As of 2024, it remains a top-selling respiratory drug in public hospitals and is included in national procurement programs. It is available through hospital pharmacies and authorized medical channels, not over-the-counter or general retail.
Details on China Manufacturer and Product: 注射用西维来司他钠 - 上海汇伦医药股份有限公司
4.2 Doxycycline and other tetracyclines
- Doxycycline at sub-antimicrobial doses is used in periodontal disease and some aneurysm/MMP-driven conditions; it inhibits MMPs more than neutrophil elastase, but net effect is ECM-protective.
- Chronic use has tolerability and microbiome issues; again, no hard data on systemic EDP.
4.3 Inhaled or local elastase inhibitors
- Various inhaled NE inhibitors have been trialed in CF/COPD; thus far, no robust clinical success.
4.4 Realistic longevity stance
Right now, no approved drug is suitable for chronic systemic NE inhibition as a longevity strategy.
5. Nutraceuticals / polyphenols / peptides that modulate NEU1 or the elastin receptor?
5.1 NEU1-selective inhibitors (not nutraceuticals)
- Medicinal chemistry has produced NEU1-selective DANA derivatives, e.g. C9-butyl-amide-DANA, which blocks NEU1 activity and NEU1-mediated responses in human lung cells and mouse lung models.
- Newer work (2022–2025) reports additional NEU1-biased sialidase inhibitors with IC₅₀ in the low micromolar range.
- All are research compounds, not supplements or drugs.
5.2 Interfering peptides / biologics
- NEU1-targeting peptides have been used to inhibit NEU1 activity in insulin signaling and other pathways in animals.
- Again: purely experimental.
5.3 Polyphenols etc.
- Some polyphenols (EGCG and others) show weak, broad glycosidase inhibition in vitro at millimolar concentrations—far above what you achieve with oral dosing. No credible evidence they meaningfully inhibit NEU1 in vivo.
- Anything claiming to be a “NEU1 inhibitor supplement” right now is marketing, not science.
So: there are no nutraceutical-grade NEU1 inhibitors with convincing data. The real action is in DANA derivatives and NEU1-specific medicinal chemistry, all preclinical.
6. Downstream biomarkers accessible today
You can’t yet buy a NEU1 or EDP panel from Quest/Labcorp, so you lean on downstream and adjacent markers.
Reasonable proxy/read-out markers :
-
hs-CRP: standard low-grade inflammation marker; not specific, but robust and cheap.
-
GlycA (NMR glycoprotein acetyls): integrated marker of systemic inflammation and subclinical vascular inflammation, predictive of cardiometabolic risk and CVD events.
-
Neutrophil elastase activity: some research labs and niche services offer NE activity assays in plasma or BAL; not widely available clinically.
-
Monocyte/NLR metrics :
- CBC-derived neutrophil-to-lymphocyte ratio (NLR) as a crude inflammaging marker.
- Flow cytometry panels (research setting) for inflammatory monocyte subsets (CD14⁺⁺CD16⁺⁺, etc.) and T-cell phenotypes (CD28null, KLRG1⁺, etc.).
-
Arterial stiffness :
-
Pulse wave velocity (PWV) and central aortic pressure; elastin degradation is a major contributor.
-
Multi-omics clocks :
- Proteomic clocks that are enriched for ECM and inflammatory proteins; the organ-specific proteomic aging clocks you’ve already looked at are conceptually compatible with this axis.
None of these are specific to ELN/NEU1, but if you’re trying to see whether a regimen is reducing chronic vascular and myeloid inflammation, they are reasonable surrogates.
8. Could intermittent senolytics (D+Q) lower elastin fragmentation in humans?
Mechanistically plausible; empirically unproven.
What we know:
- In mice, D+Q reduced senescent myeloid cells and elastase activity and lowered elastin fragments. That’s directly in the paper.
- In humans, small trials show that intermittent D+Q:
- reduces senescent cell markers and SASP factors in adipose tissue and blood,
- improves some functional measures (e.g., mobility, bone metabolism).
-
No published human data yet on EDP/ELN-fragment levels before/after senolytic treatment.
So, it is reasonable to hypothesize that effective senolysis will indirectly reduce elastase load and elastin fragmentation over time—but we don’t have measurements. Until we see actual EDP or ECM-related imaging endpoints in senolytic trials, it’s an open question.
9. At what age does ELN-fragment accumulation become relevant for people?
From the Nature Aging paper’s human cohort:
- ELN fragments increase with chronological age across adult life and correlate with worse cardiometabolic and renal markers and higher blood pressure. Exact age cut-points in that dataset are not fully detailed in the abstract, but the pattern is monotonic.
- Other ECM-related changes (arterial stiffness, PWV, collagen/elastin remodeling) start to diverge measurably from early to mid-adulthood, especially in people with hypertension, obesity, or smoking history.
Reasonable inference:
- For a metabolically healthy, normotensive, non-smoker, early 20s–30s probably have low EDP load.
- By 30s–40s, lifestyle and risk factors start to diverge, likely producing different EDP trajectories.
- By 50+, EDP is almost certainly relevant as a systemic biomarker of vascular and organ aging.
But since there are no public EDP reference curves by age, we can’t draw a clean line like “age 35 is when you must intervene.” The rational stance: treat ECM-sparing behaviors as life-long, not late-life rescue.
10. Realistic timelines for NEU1-selective therapeutics
State of the field:
- Recent reviews emphasize NEU1 as a target in fibrosis, cardiovascular disease, and inflammation, and summarize NEU1-selective inhibitors (e.g., C9-butyl-amide-DANA) with in vitro and in vivo lung data.
- 2022–2025 medicinal chemistry papers report newer NEU1-biased compounds with micromolar potency, but no human PK, no toxicity, no phase I yet.
Drug-development reality:
- From “tool compound with animal efficacy” → “approved human drug” is typically 10–15 years if everything goes smoothly (and many fail).
- NEU1 is also lysosomal and membrane-associated, raising complexities in achieving tissue-specific, safe inhibition.
So best guess :
- 0–5 years: more animal data (fibrosis, cardiovascular, metabolic, possibly aging) + first-in-human safety studies if a candidate looks clean.
- 5–10+ years: if successful, early disease-specific applications (pulmonary fibrosis, NASH, maybe vascular remodeling).
- Aging/geroprotection as a primary indication would likely be even further out, if it happens at all.