Part 4: Actionable Intelligence
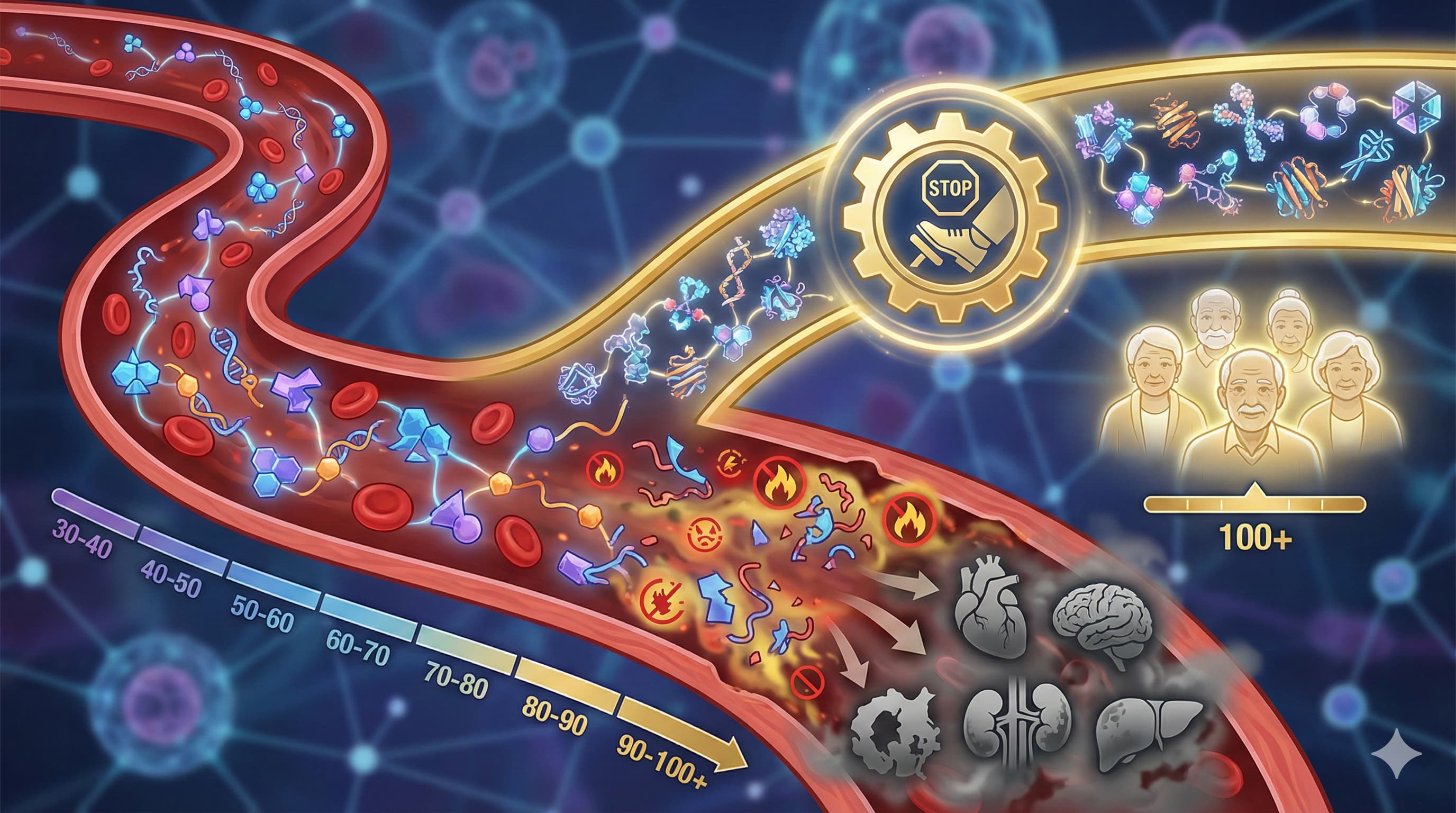
The Translational Protocol: Centenarian Mimicry via DPP4 Inhibition
Target: Dipeptidyl Peptidase-4 (DPP4). Logic: Centenarians maintain low DPP4 levels. High DPP4 accelerates vascular aging and fibrosis. Candidate Agent: Sitagliptin (Januvia) or Teneligliptin (Generic available).
1. Human Equivalent Dose (HED) Calculation
-
Source Data: Murine studies utilize Sitagliptin at ~100–250 mg/kg/day for maximal GLP-1 effect, but “healthspan” effects (weight/adiposity reduction) are seen at 0.4% w/w diet, roughly equivalent to ~40–50 mg/kg/day in mice.
-
Formula: HED(mg/kg)=AnimalDose(mg/kg)×HumanKmAnimalKm
-
Calculation:
- 50mg/kg×(3/37)≈4.05mg/kg
- For a 75 kg human: 4.05×75=303mg (Daily).
-
Correction Factor: FDA approved human maintenance dose for T2D is 100 mg/day. The murine “longevity” doses are often supratherapeutic.
-
Recommended Protocol Dose: 25–50 mg/day (Low-dose intermittent).
-
Reasoning: You are mimicking a “lower baseline” (Centenarian profile), not treating acute hyperglycemia. 100 mg/day inhibits >80% of plasma DPP4 for 24 hours. A lower dose (25–50 mg) provides partial suppression (~40–60%), avoiding complete enzymatic blockade which may be immunosuppressive.
2. Pharmacokinetics (PK/PD)
-
Bioavailability: ~87% (High oral absorption).
-
Half-life (t1/2): ~12.4 hours (Supports once-daily dosing).
-
Metabolism: Primarily renal excretion (79% unchanged). Minimal CYP450 involvement (Low risk of drug-drug interactions).
3. Safety & Toxicity Profile
4. Biomarker Verification (Proof of Efficacy)
To verify you are achieving “Centenarian Mimicry” without over-suppression:
-
Primary: Serum DPP4 Activity (Commercial assay available). Target: Reduction to ~50% of age-matched baseline.
-
Secondary: HbA1c (Should drop slightly or stabilize <5.4%).
-
Downstream: GLP-1 (Active) levels should rise 2-fold post-meal.
5. Feasibility & ROI
-
Sourcing:
-
Rx: Sitagliptin (Januvia) is expensive ($500+/month).
-
Generic (Grey Market/Off-shore): “Zituvio” or Indian generics cost ~$0.50 – $1.00 per tablet.
-
Cost vs. Effect:
-
Generic: ~$15–30/month. High ROI if it mimics GLP-1 agonist effects (weight/inflammation control) at 1/10th the cost of Semaglutide.
Part 5: The Strategic FAQ
1. “Is the ‘Centenarian Signature’ a cause of longevity or just a survivor effect?”
Answer: Likely a mix, but the GDF15 data strongly suggests it’s a survivor effect (resistance to stress). However, the DPP4 and FASLG data imply a causal mechanism: these proteins actively drive tissue deterioration. If you have high DPP4 at 50, you are accelerating your own aging clock.
2. “Why not just take Metformin? It hits similar pathways (AMPK).”
Answer: Metformin is a “dirty” drug that hits Complex I and alters the gut microbiome. The centenarian data highlights FASLG (Apoptosis) and ECM (Fibrosis) pathways, which Metformin touches only indirectly. This suggests a need for specific anti-fibrotic support (like DPP4 inhibitors) alongside metabolic agents.
3. “Does this validate ‘Young Blood’ (Parabiosis) transfusions?”
Answer: Yes, obliquely. The fact that centenarian plasma lacks “pro-aging” factors (like high FASLG) supports the idea that diluting old plasma (plasmapheresis) might be as effective as adding young factors. You are removing the “brakes” (inhibitory proteins).
4. “How does GDF15 ‘break the curve’?”
Answer: In normal aging, GDF15 rockets up linearly as mitochondria fail. In centenarians, this rise slows down. This implies their mitochondria are either more resilient or they have better “mitophagy” (cleanup) that prevents the GDF15 distress signal from spiking.
5. “Is the FOXO3 link actionable without gene editing?”
Answer: Yes. Intermittent Fasting and Heat Stress (Sauna) are proven phenotypic activators of FOXO3. The study confirms that keeping FOXO3 active is the goal. You don’t need a CRISPR edit; you need lifestyle stress-mimetics.
6. “What about the 80-year-old controls? Were they too sick?”
Answer: Yes. The “Geriatric” group was hospitalized. This is a major confounder. The “Centenarian Signature” might partially be a “Non-Hospitalized Signature.” However, the magnitude of the protein differences (Log2FC > 1.5) is too large to be explained by acute illness alone.
7. “Can I measure these 37 proteins myself?”
Answer: Not easily. The Olink panel is a research tool ($$$). However, you can measure GDF15, HbA1c, Cystatin C(kidney function), and hs-CRP. If these are low, you are likely suppressing the “inflammaging” cluster identified in the paper.
8. “Is there a sex difference in this signature?”
Answer: Most centenarians are female (survivorship bias). The study did not power sufficiently to separate male vs. female signatures. The “Life Extension” effect of similar pathways (like mTOR inhibition) often shows sex-dimorphism in mice (females benefit more).
9. “Does Sitagliptin conflict with Rapamycin?”
Answer: No. In fact, they may be synergistic. Rapamycin inhibits mTOR (growth), while Sitagliptin boosts GLP-1 (insulin sensitivity). A 2024 mouse study found the combination of Rapamycin + Trametinib (another pathway) extended life 30%. Combining Rapamycin + DPP4i is a logical, non-overlapping stack.
10. “What is the single most accessible intervention from this paper?”
Answer: Strict Glucose/Insulin Control. The dominance of the insulin/IGF-1/ECM cluster (DPP4, GLO1, insulin-like proteins) screams that metabolic preservation is the gatekeeper to age 100. If you can’t get Sitagliptin, get your fasting insulin < 5 uIU/mL.