BioAge Announces Additional Positive Interim Phase 1 Data for BGE-102, a Novel Brain-Penetrant NLRP3 Inhibitor, Demonstrating Potential for Best-in-Class hsCRP Reduction in Participants with Elevated Cardiovascular Risk
January 12, 2026
First BGE-102 MAD cohort completed in obese individuals with elevated hsCRP receiving 120 mg QD; demonstrated rapid and profound reduction in inflammatory markers
BGE-102 achieved 86% reduction in hsCRP at Day 14, with 93% of participants reaching normalized levels (<2 mg/L)
BGE-102 demonstrated significant reductions in IL-6, a key driver of systemic inflammation and cardiovascular risk, and fibrinogen, an independent predictor of cardiovascular events
BGE-102 was well tolerated with a favorable safety profile
Patent issued covering additional composition of matter and novel NLRP3 binding site
Full Phase 1 data, including additional MAD cohorts in obese participants with elevated hsCRP, anticipated 1H 2026; Phase 2a study on track to initiate in 1H 2026
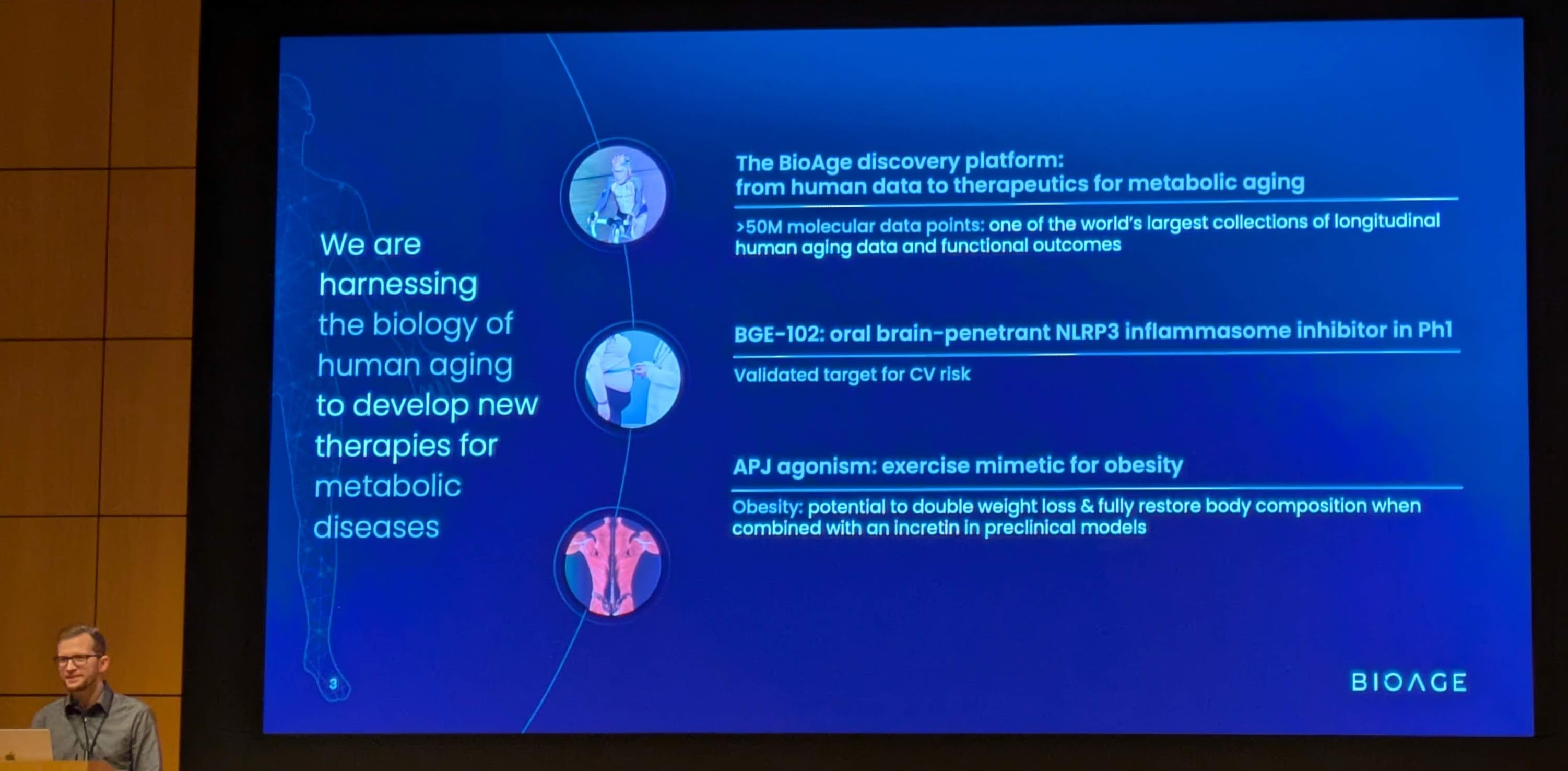
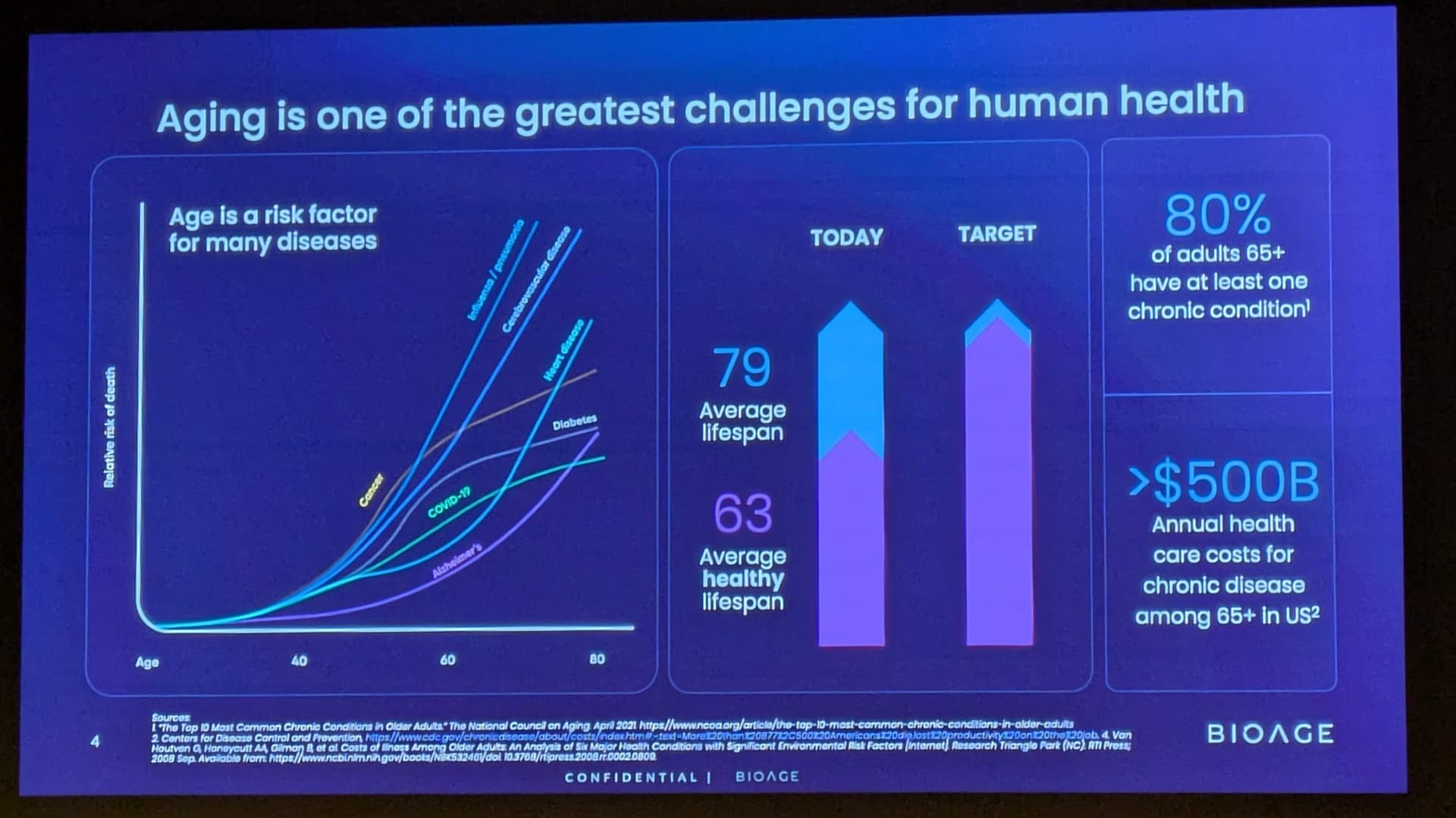
EMERYVILLE, Calif., Jan. 12, 2026 (GLOBE NEWSWIRE) – BioAge Labs, Inc. (Nasdaq: BIOA) (“BioAge”), a clinical-stage biopharmaceutical company developing therapeutic product candidates for metabolic diseases by targeting the biology of human aging, today announced additional positive interim data from the ongoing Phase 1 clinical trial evaluating BGE-102, a potent, structurally novel, orally available, brain-penetrant small molecule NLRP3 inhibitor being developed for the treatment of patients with cardiovascular risk factors.
In a multiple ascending dose (MAD) cohort of participants with obesity (BMI 32–42) and elevated baseline inflammation (hsCRP >3 mg/L), BGE-102 120 mg once daily achieved an 86% median reduction in high-sensitivity C-reactive protein (hsCRP) at Day 14. Notably, 93% of BGE-102-dosed participants (13 of 14) achieved hsCRP levels below 2 mg/L—the clinically recognized threshold for reduced cardiovascular risk.
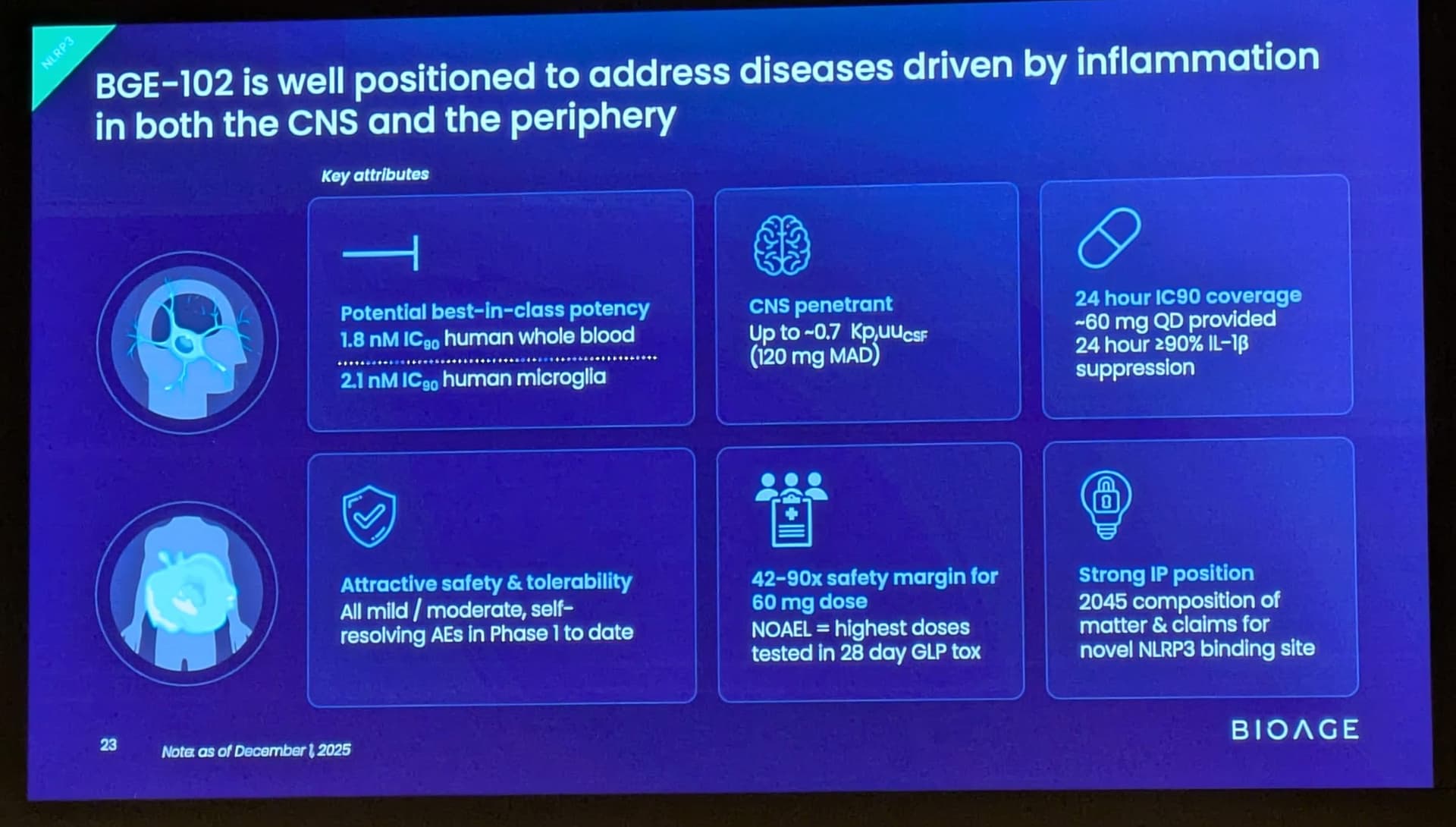
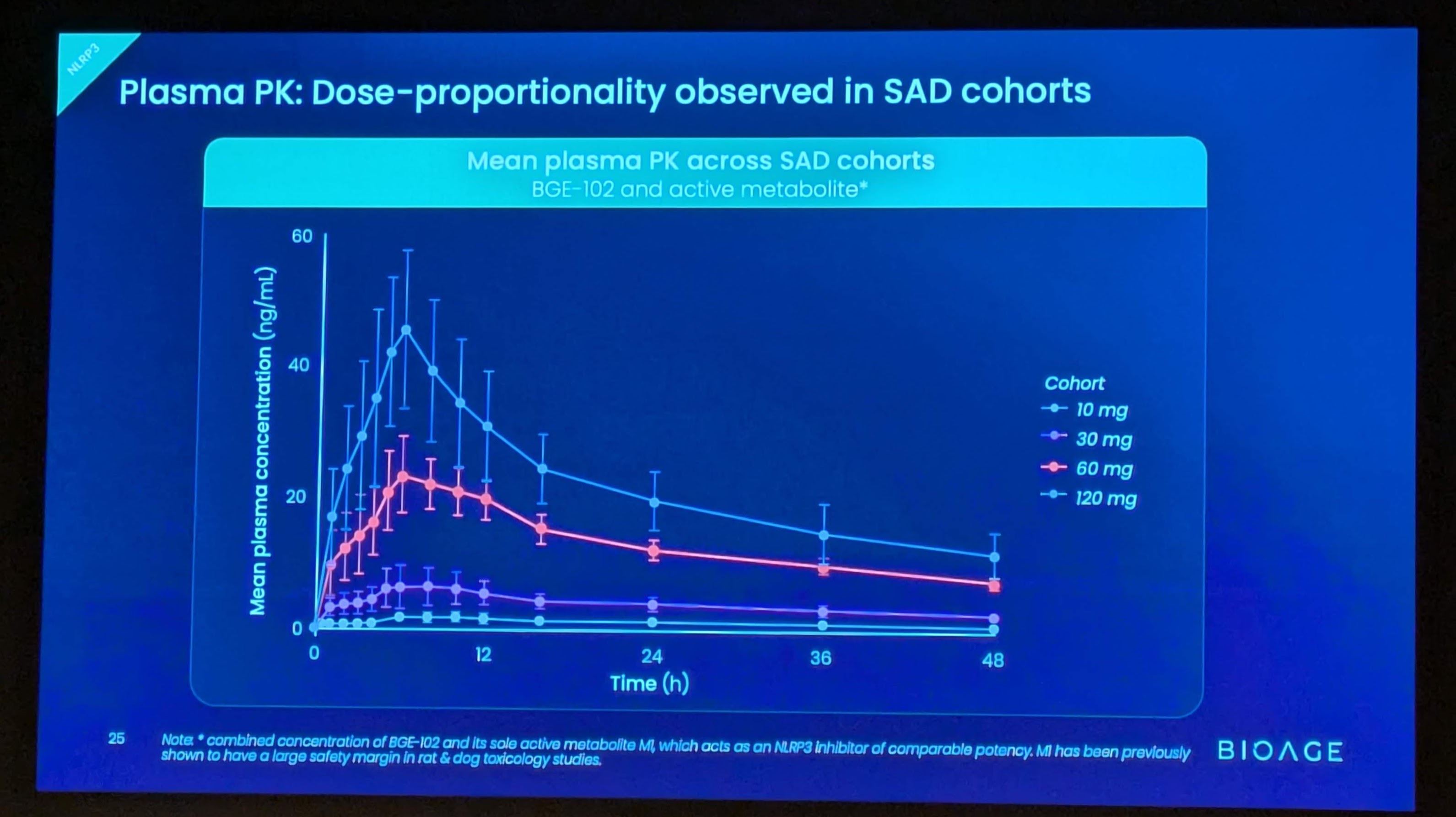
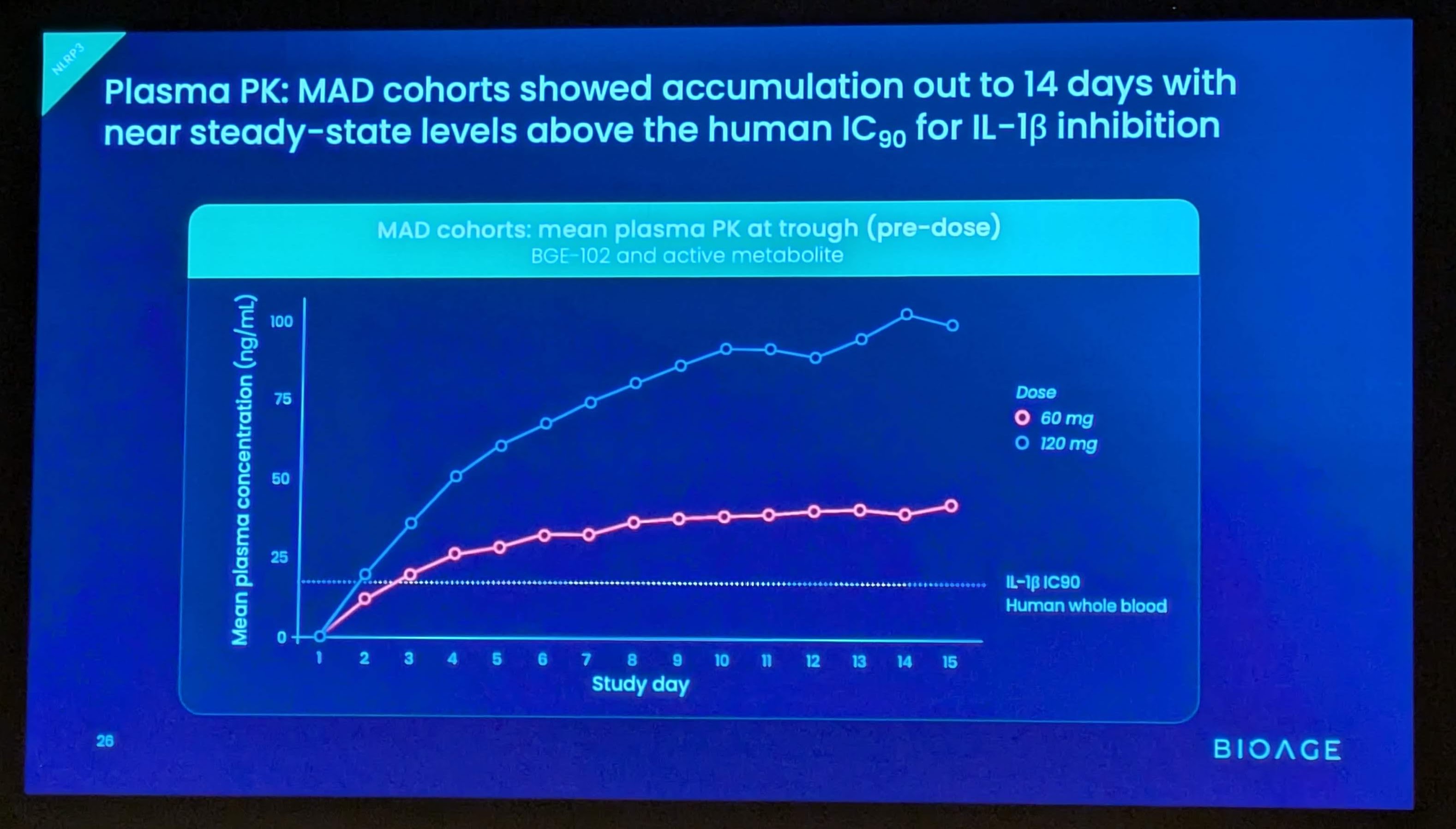
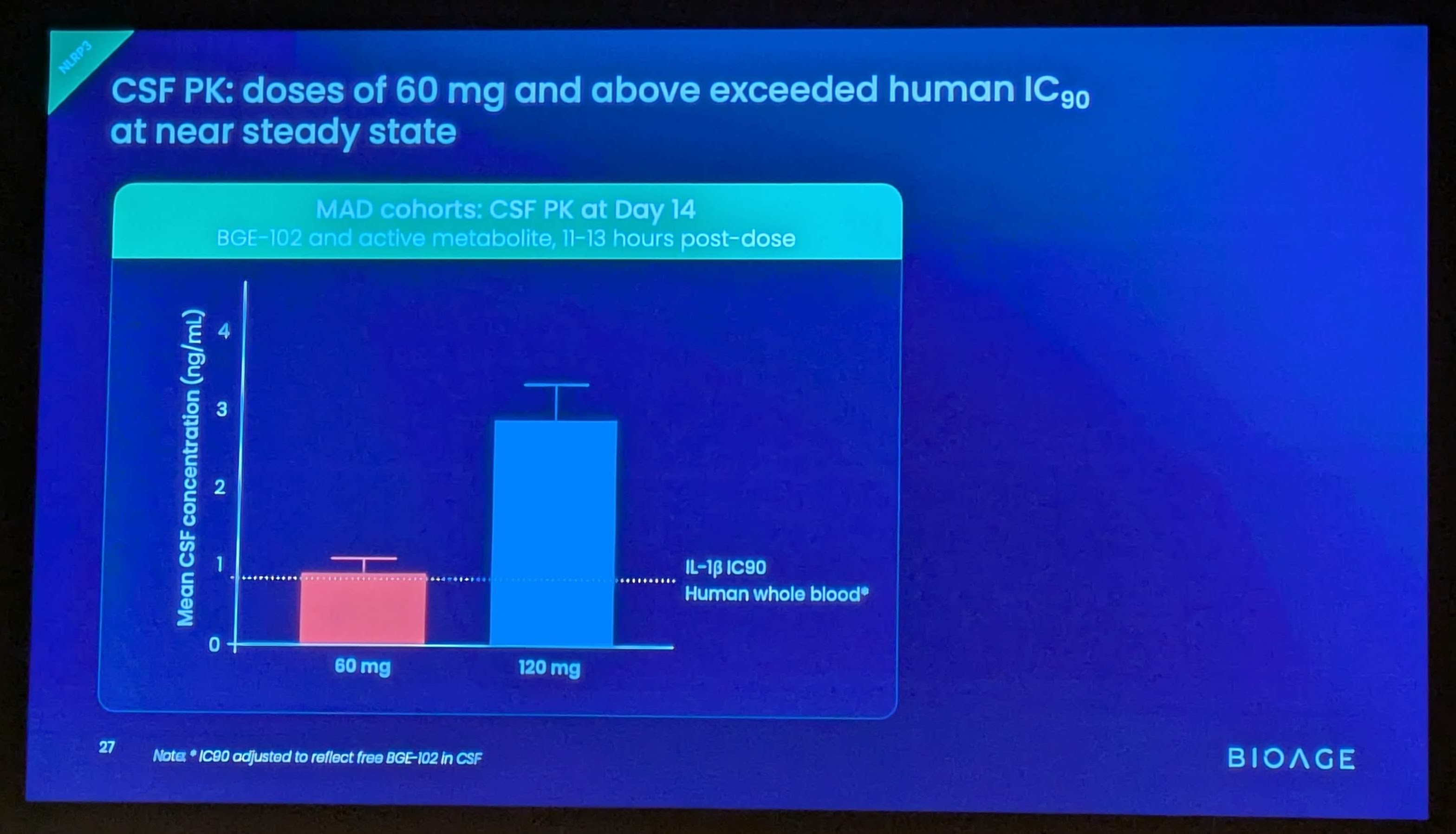
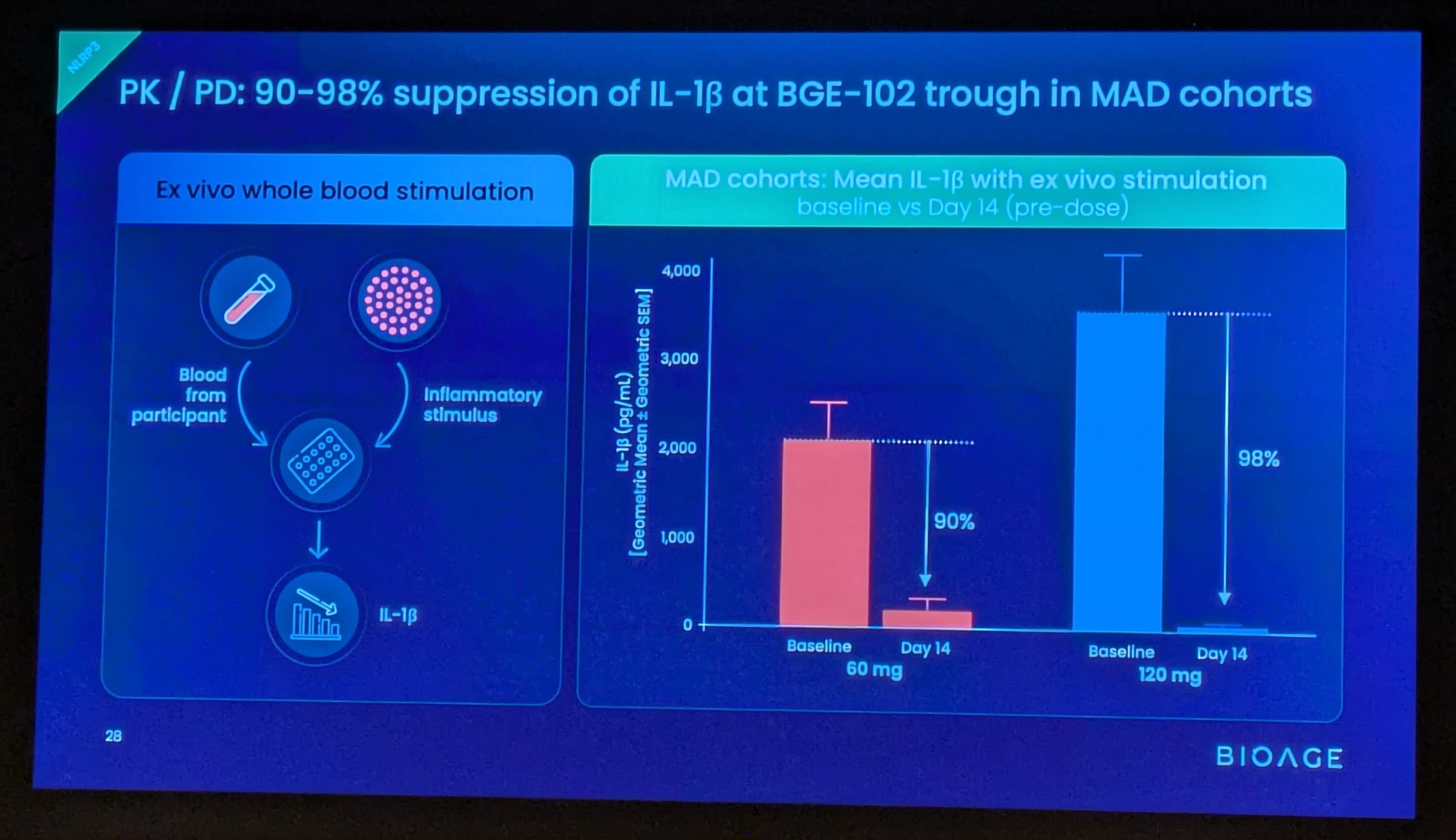
These findings build on positive interim data announced in December 2025 from SAD and initial MAD cohorts, which demonstrated that BGE-102 was well tolerated, achieved dose-proportional pharmacokinetics supporting once-daily dosing, and produced 90-98% suppression of IL-1β at Day 14 trough. Those data also confirmed high brain penetration, with cerebrospinal fluid (CSF) concentrations exceeding the IC90 at doses of 60 mg and above.
“We are very encouraged by these results, which support the potential for BGE-102 to deliver injectable-like inflammation reduction in an oral therapy designed for primary care, the clinical setting where most cardiovascular risk is managed and where oral medicines are preferred by patients and physicians,” said Kristen Fortney, PhD, CEO and co-founder of BioAge. “Chronic inflammation is now recognized as a major driver of cardiovascular disease—on par with cholesterol—yet it remains far less commonly treated. An 86% reduction in hsCRP, with 93% of participants reaching levels associated with reduced cardiovascular risk, positions BGE-102 as a potential best-in-class oral therapy to directly address inflammation. These findings support our plans to advance BGE-102 into a Phase 2a study in the first half of this year.”
Key findings from the MAD cohort in patients with obesity and elevated hsCRP
Rapid and profound hsCRP reduction
- BGE-102 achieved 83% median reduction in hsCRP (from a median baseline of 4.85 mg/L) at Day 7 and 86% at Day 14
- 93% of participants (13/14) on BGE-102 achieved hsCRP <2 mg/L at Day 14; 71% (10/14) reached ≤1 mg/L
- Rapid onset of effect: 86% of BGE-102-dosed participants (12/14) achieved hsCRP levels <2 mg/L at Day 7; 71% (10/14) reached ≤1 mg/L
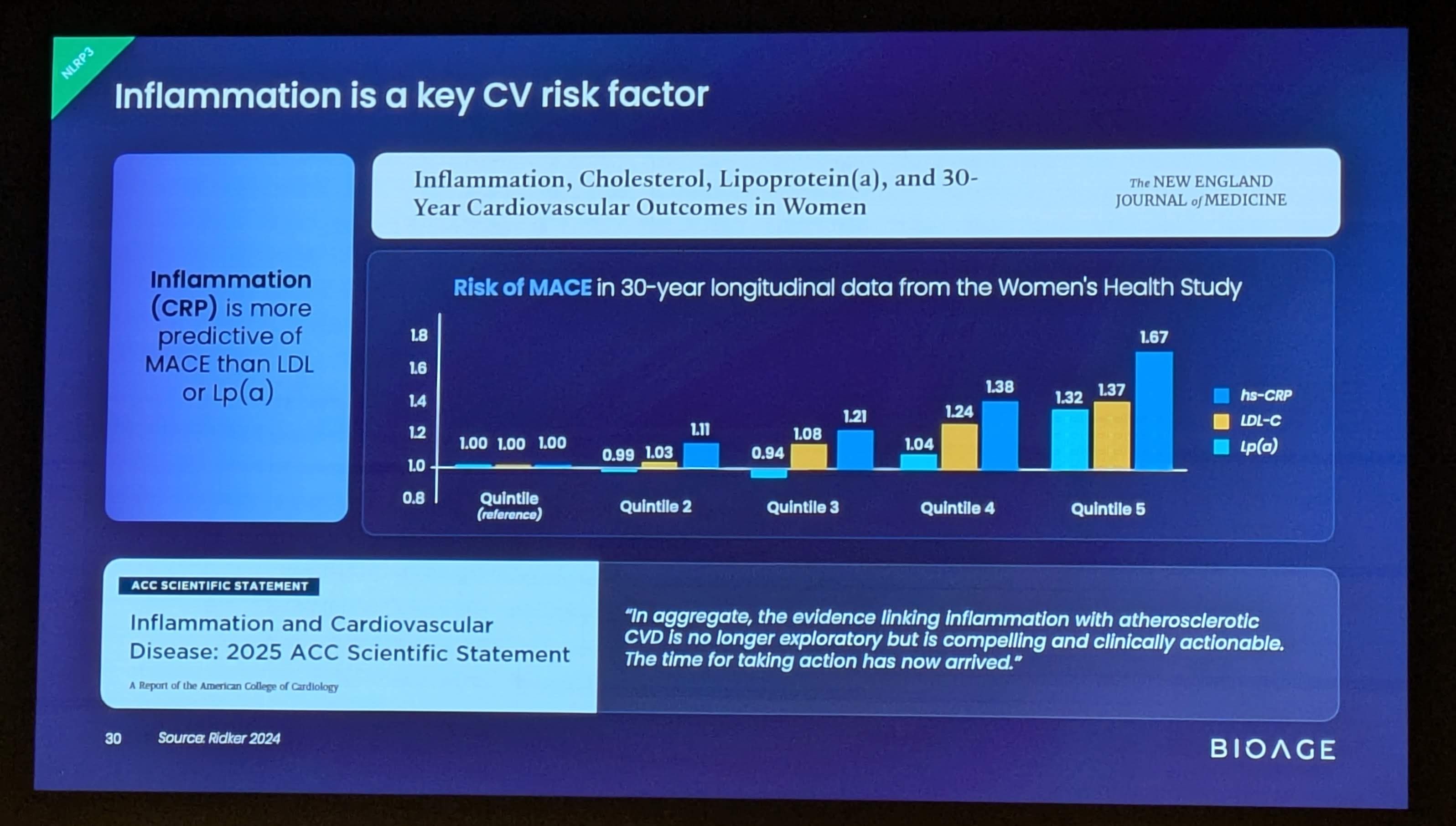
- hsCRP is the most widely used marker of inflammatory cardiovascular risk; levels below 2 mg/L are associated with reduced risk of cardiovascular events
Significant IL-6 reduction
- BGE-102 achieved a 44% median reduction in serum IL-6 at Day 14
- CSF IL-6 decreased in the two participants with elevated baseline levels, consistent with BGE-102’s high brain penetration
- IL-6 is a key upstream driver of hsCRP production and a validated marker of cardiovascular risk
Significant fibrinogen reduction
- BGE-102 achieved a 30% reduction in fibrinogen at Day 14
- Elevated fibrinogen has been shown to be an independent predictor of cardiovascular events and thrombotic risk
Potent IL-1β suppression, consistent with strong target engagement
- In the ex vivo whole blood stimulation assay, BGE-102 achieved 93% suppression of IL-1β at trough (Day 14, pre-dose)
- IL-1β is directly downstream of NLRP3 and drives production of IL-6 and CRP, key markers of cardiovascular risk
Safety and tolerability
- BGE-102 continued to be well tolerated
- Adverse events were infrequent, mild to moderate in severity, and self-limited, with no dose-dependent pattern observed
- No dose-limiting toxicities observed
Additional figures and data from the ongoing Phase 1 study are available in the Company’s corporate presentation at https://ir.bioagelabs.com/.
“The substantial reductions in hsCRP, IL-6, and fibrinogen we observed in participants with obesity and elevated inflammation demonstrate that BGE-102 potently suppresses the NLRP3-driven inflammatory cascade in a clinically relevant population,” said Paul Rubin, MD, Chief Medical Officer of BioAge. “These data provide strong rationale for advancing into our planned Phase 2a study, where we will evaluate BGE-102’s effects on a range of key inflammatory biomarkers over a longer duration in patients with elevated cardiovascular risk.”
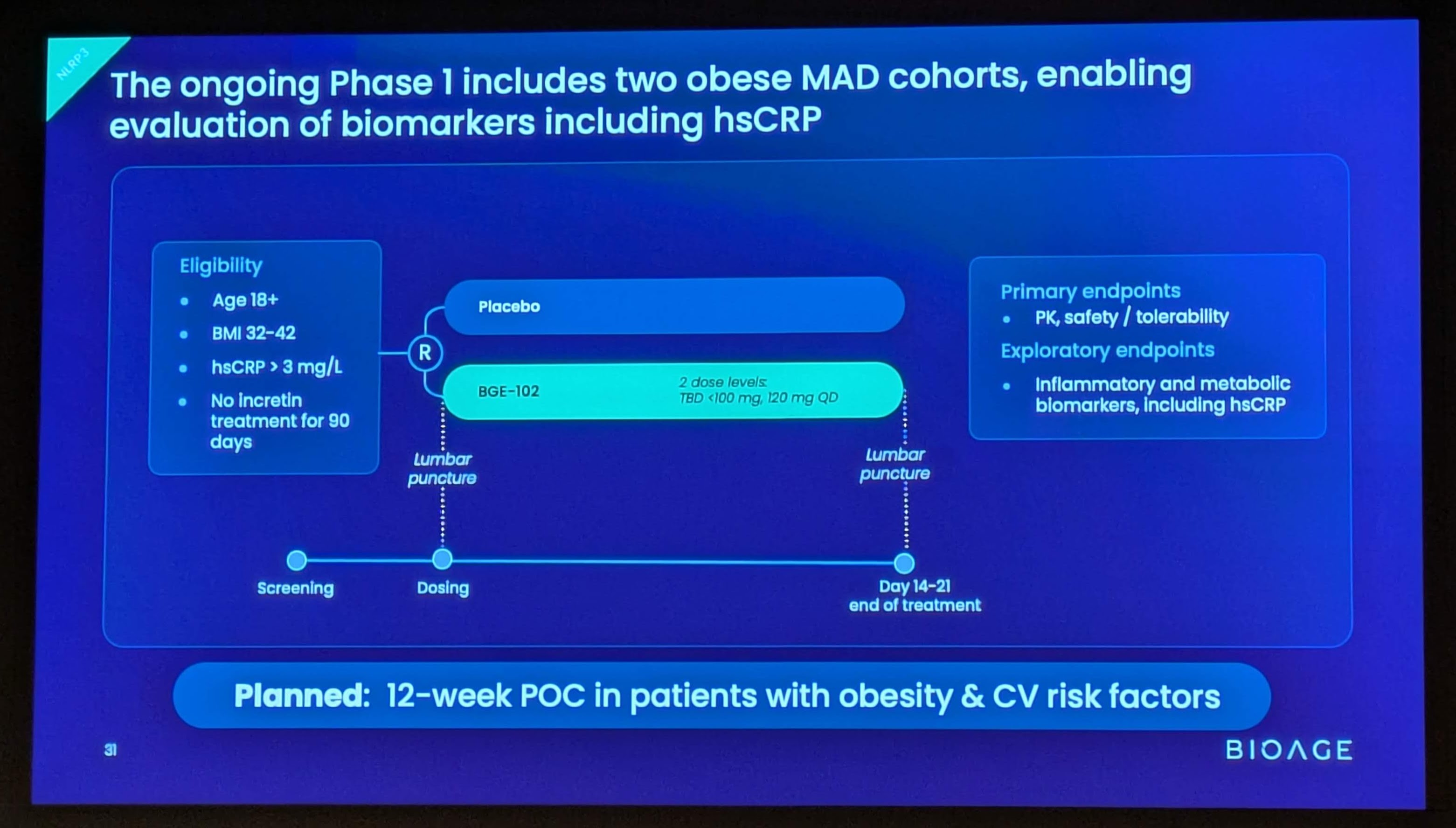
Phase 1 study design
The ongoing Phase 1 study is a randomized, double-blind, placebo-controlled trial in healthy volunteers and participants with obesity. Part 1 evaluated single ascending doses at four dose levels (10, 30, 60, and 120 mg); Part 2 to date has evaluated multiple ascending doses administered once daily for 14 days in healthy volunteers (60 and 120 mg) and in participants with obesity and elevated hsCRP (120 mg QD cohort complete; two lower-dose QD cohorts ongoing). Pharmacodynamic effects were evaluated by assessment of serum biomarkers including hsCRP, IL-6, and fibrinogen, as well as an ex vivo whole blood stimulation assay measuring IL-1β suppression.
Anticipated milestones for BGE-102 in cardiovascular disease
-
1H 2026: Completion of Phase 1 trial with full data readout, including two additional MAD cohorts in obese participants with elevated hsCRP
-
1H 2026: Initiation of Phase 2a proof-of-concept study in patients with obesity and cardiovascular risk factors. The trial is planned to enroll approximately 100 patients randomized 1:1 to BGE-102 monotherapy or placebo for 12 weeks. The anticipated primary endpoint is percent change in hsCRP. The trial will also assess inflammatory and metabolic biomarkers, and will include liver MRI.
-
2H 2026: Phase 2a data readout
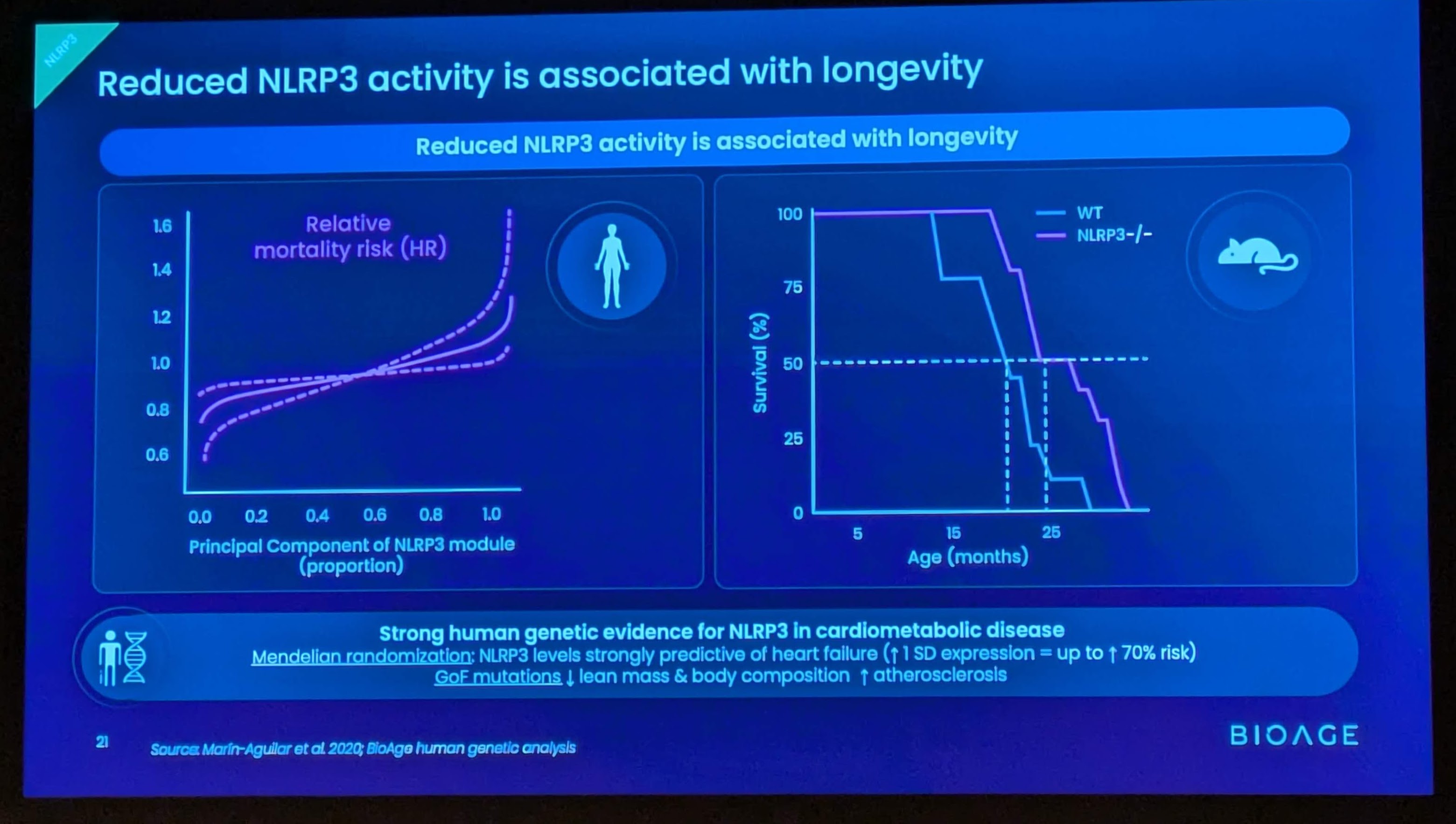
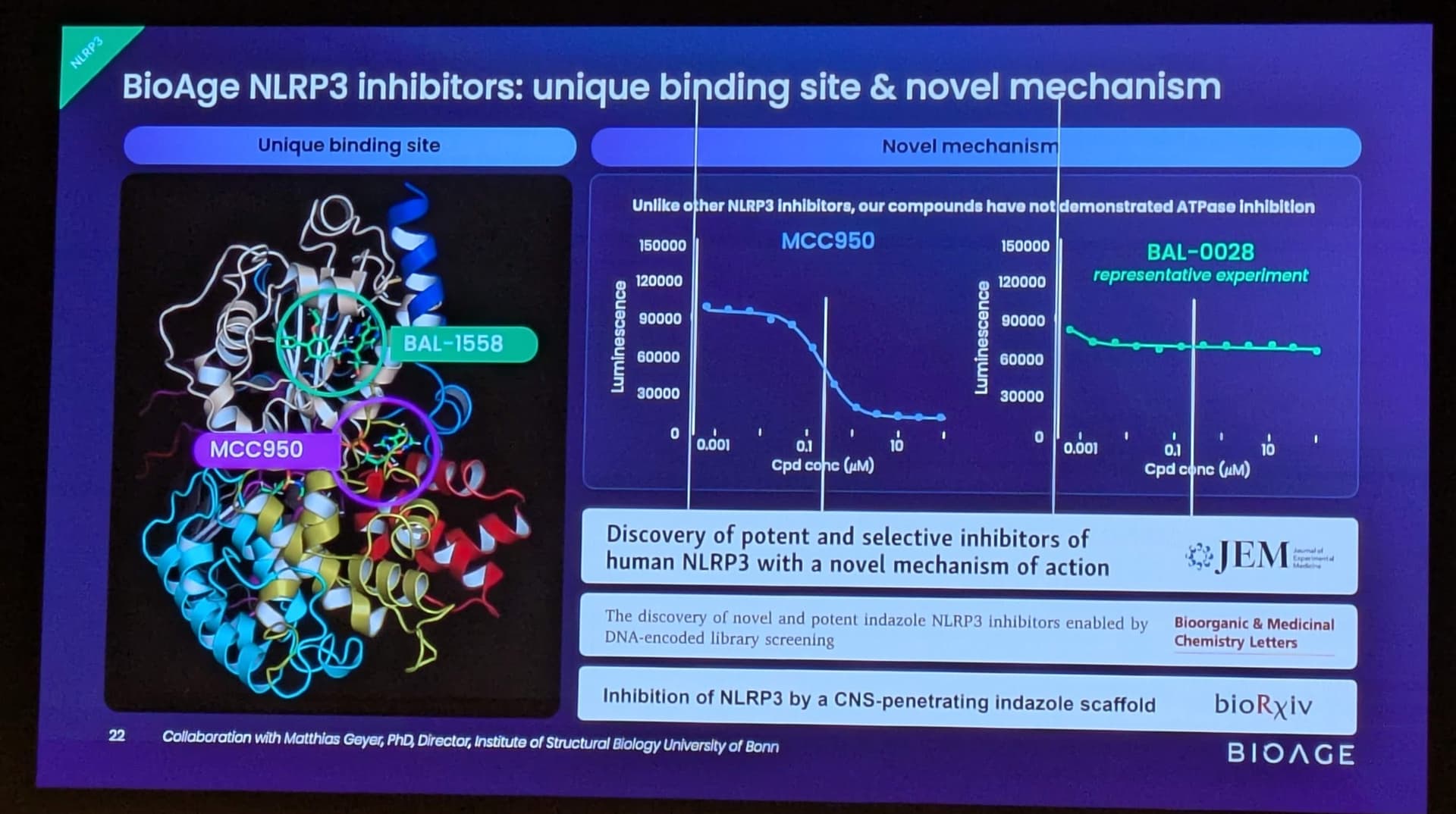
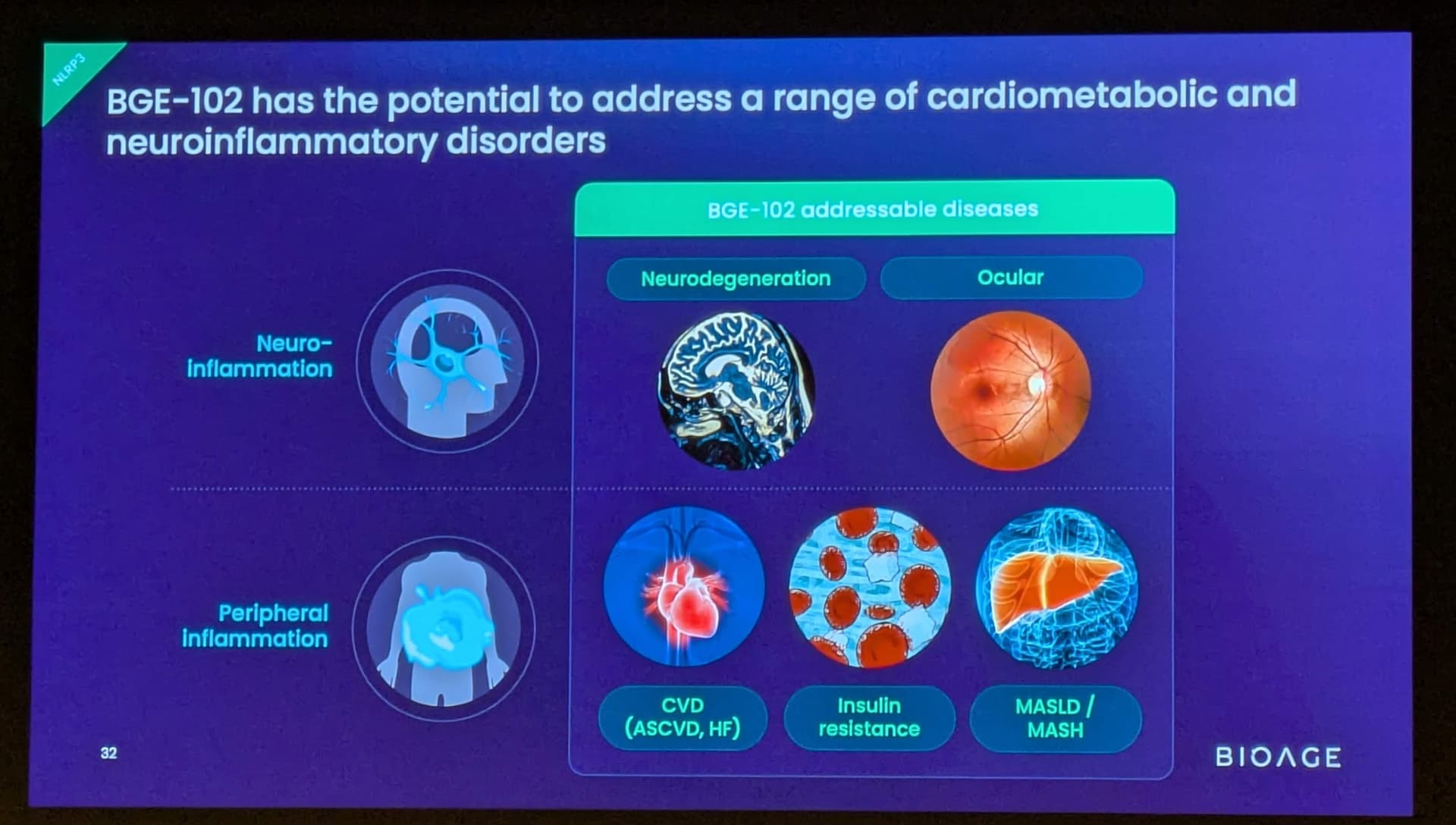
Background on BGE-102 and NLRP3
BGE-102 is a potent, orally available, brain-penetrant small molecule NLRP3 inhibitor being developed for diseases of inflammation including elevated cardiovascular risk. BGE-102 represents a structurally novel class of NLRP3 inhibitors developed by BioAge with a unique mechanism and binding site. NLRP3 is a key driver of age-related inflammation that has been implicated in a broad range of diseases, including cardiovascular disease, neurodegeneration, and metabolic disorders.