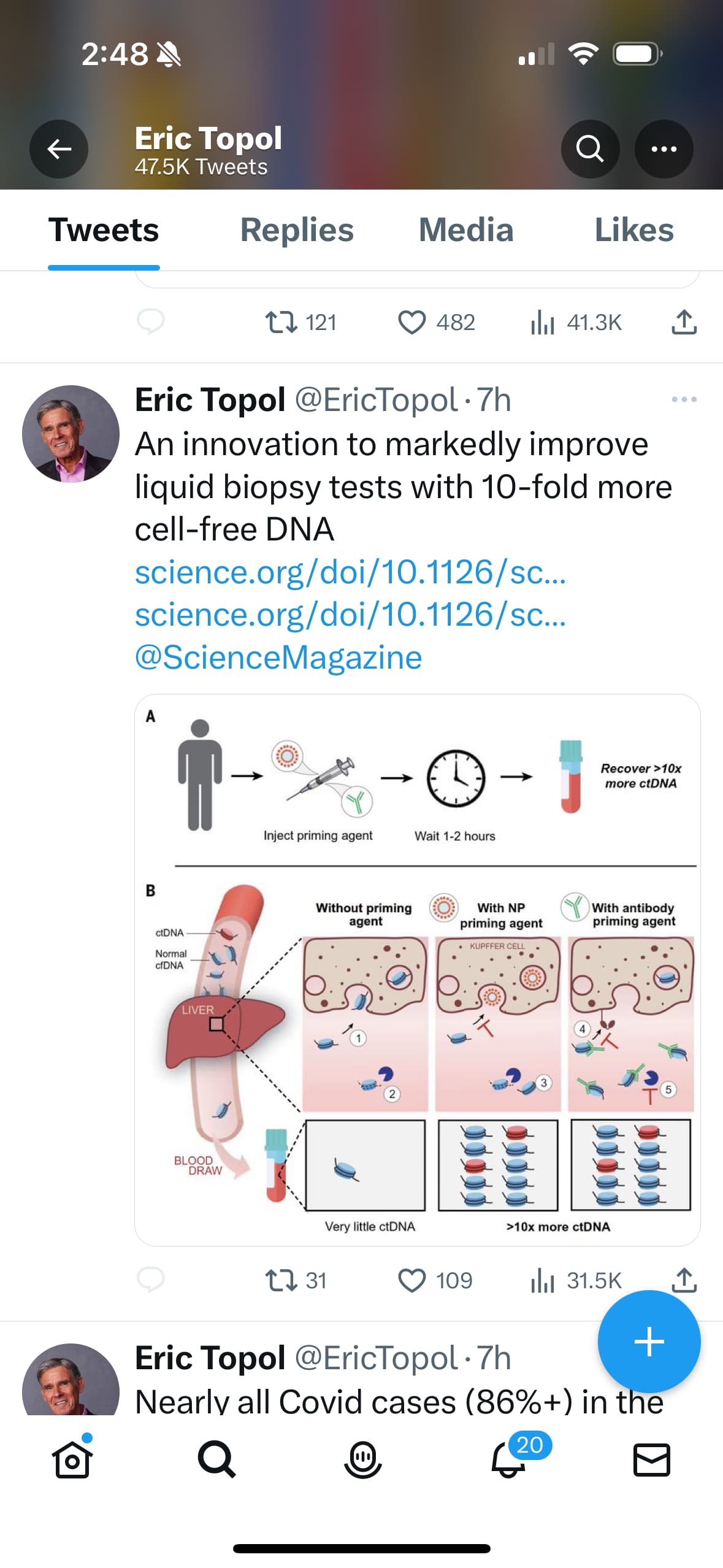
Galleri, from a company called Grail, is just one of a number of new tests seeking to detect multiple cancers very early on. It’s probably the buzziest of this new class — in part because it’s the most widely available and also because it’s at the center of a cross-Atlantic antitrust battle that started when DNA-sequencing giant Illumina moved to acquire Grail in 2021.
The promise of such tests is great: Patient survival rates are much higher when cancers are detected early.
“Someday this will become routine for people who are at high risk,” Eric Topol, director of Scripps Research Translational Institute, tells me. “But we’re not there yet*.”*
The Grail test is already commercially available. But all of these tests will require more study to show what they do best, who’s most likely to benefit and how good they are at actually preventing deaths. The UK’s National Health Service is partnering with Grail to evaluate just such questions.
Another thing that will become clearer with more time and data, says Topol, is the best approach for detecting the signals of early-stage cancer and then figuring out where it is in the body. Grail and its competitors, like Thrive (Thrive was acquired by Exact Sciences in 2020), have taken pretty drastically different approaches to doing this. The best test, Topol tells me, might actually be one that combines several different approaches.
Full writeup here: https://archive.ph/H1TPc
Links to the two Cancer Screening Companies:
Exact Sciences
Galleri
Related Reading:
Exact Sciences says its new colon cancer test shows 30% lower false positive rate
Multi-cancer early detection test in symptomatic patients referred for cancer investigation in England and Wales (SYMPLIFY): a large-scale, observational cohort study
Interpretation
This first large-scale prospective evaluation of an MCED diagnostic test in a symptomatic population demonstrates the feasibility of using an MCED test to assist clinicians with decisions regarding urgency and route of referral from primary care. Our data provide the basis for a prospective, interventional study in patients presenting to primary care with non-specific signs and symptoms.
Open Access/Full Paper:
https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(23)00277-2/fulltext
and in the “oops” department:
Roughly 400 patients who agreed to take cancer detection tests with biotech firm Grail received letters indicating they may have cancer, but the company said the letters were not accurate, blaming it on a “software configuration issue” that caused its telemedicine vendor to send out the incorrect information—a development that’s caused at least one life insurance company to reevaluate its business with the cancer screening firm, according to the Financial Times.
What to Know Now: Commercial blood tests are one tool to help detect pancreatic cancer at an earlier stage. These tests detect certain markers or compounds in the blood that may suggest cancer is present. Several now on the market are multi-cancer tests, like the Galleri test from GRAIL and OneTest™, a multi-cancer test from 20/20 GeneSystems that looks for certain proteins that may signal the presence of cancer.
The Avantect Pancreatic Cancer Test from ClearNote Health focuses specifically on pancreatic cancer. It uses a blood sample to detect a biomarker called “circulating free DNA.” A negative test result means that this biomarker has not been detected. A positive test result means that this biomarker was detected, which could mean a person has pancreatic cancer, or other conditions such as pancreatitis or intraductal papillary neoplasms (IPMNs, a type of cyst of the pancreas).
It’s important to note that a blood test cannot say with certainty whether a person has pancreatic cancer. Follow-up testing such as imaging and a biopsy are necessary. Since many blood tests are expensive and not always covered by insurance, the first step is to have a conversation with your doctor about your risk factors and whether a blood test would be helpful.