Thanks. This year I ordered OneTest for the reason stated. At my appointment with my GP next year I will inquire about Galleri and see if an age exception can be made as you suggested.
Comparing the different tests, I do not believe there is a “best option” for everyone. What is being tested varies as does the specificity and sensitivity of the output. For me that means there may be some advantages of taking multiple cancer blood tests.
Liquid biopsy-based multi-cancer early detection: an exploration road from evidence to implementation
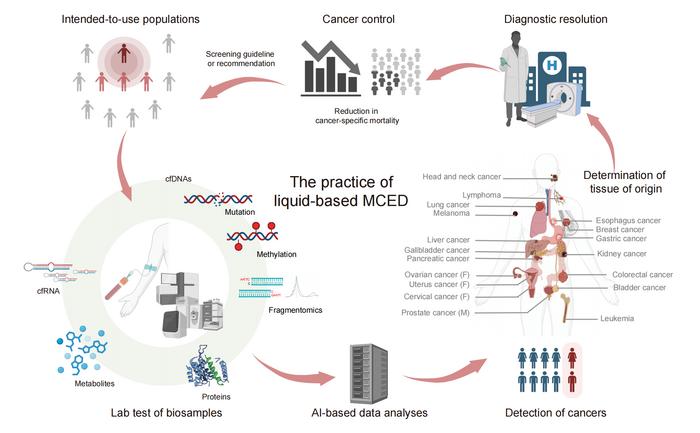
MCED is able to detect multiple cancer types by analyzing cfDNAs, cfRNAs, circulating proteins and metabolites using liquid-based biopsy techniques and AI algorithms. The TOO results of an MCED test provide further guidance for subsequent clinical diagnosis and treatment. This new screening paradigm will eventually reduce cancer-specific mortality and be recommended for a broader population.
Liquid biopsy-based multi-cancer early detection (MCED) is regarded as a breakthrough in cancer screening and prevention. With advantages such as broad population and cancer-type coverage, minimal invasiveness, high patient compliance, and favorable health economic outcomes, MCED enables the detection of multiple cancers simultaneously—potentially with tissue-of-origin localization—through the analysis of biological markers such as cfDNA, cfRNA, and proteins collected from blood or other body fluids, enhanced by AI-driven algorithms.
Although several products have advanced their MCED platforms through technical development and into clinical phases, no MCED product has yet been approved for market release globally. This indicates that the translational pathway from laboratory research to clinical implementation still requires further validation through clinical trials and real-world evidence. From the standpoint of clinical efficacy, key metrics such as sensitivity, specificity, and TOO accuracy serve as crucial evidentiary endpoints and merit in-depth investigation. From the clinical utility perspective, “reduction in late-stage cancer incidence” is increasingly recognized as a feasible and credible surrogate endpoint for “reduction in cancer-specific mortality,” given its shorter time span, lower cost, and higher practicality. Policymakers in public health must weigh a wide range of clinical evidence carefully when developing MCED-related screening guidelines.
At present, MCED should be viewed as a complementary, rather than replacement, strategy to existing cancer screening modalities.
Anyone has knowledge of or experience with the Pantum detect cancer test from zyagnum?
It’s part of my health insurance and done once a year.
Would be curious about any data or results. I haven’t found much beside from some German comments which weren’t too optimistic about it. They criticize the studies made
Blood Test Detects Head and Neck Cancer up to 10 Years Before Symptoms
Detecting cancer years before symptoms
In a newly funded federal study published in the Journal of the National Cancer Institute, researchers at Mass General Brigham demonstrated that their liquid biopsy test, called HPV-DeepSeek, can detect HPV-related head and neck cancers as early as 10 years before symptoms develop. According to the study’s authors, diagnosing these cancers earlier could increase treatment success rates and reduce the need for aggressive therapies.
“Our study shows for the first time that we can accurately detect HPV-associated cancers in asymptomatic individuals many years before they are ever diagnosed with cancer,” said lead study author Daniel L. Faden, MD, FACS, a head and neck surgical oncologist and principal investigator in the Mike Toth Head and Neck Cancer Research Center at Mass Eye and Ear, a member of the Mass General Brigham healthcare system.
“By the time patients enter our clinics with symptoms from the cancer, they require treatments that cause significant, life-long side effects. We hope tools like HPV-DeepSeek will allow us to catch these cancers at their very earliest stages, which ultimately can improve patient outcomes and quality of life.”
How HPV-DeepSeek works
HPV-DeepSeek relies on whole-genome sequencing to identify tiny fragments of HPV DNA that separate from tumors and circulate in the blood. Earlier studies by the same research groupdemonstrated that the test could reach 99% specificity and 99% sensitivity in diagnosing cancer at a patient’s initial clinic visit, performing better than existing diagnostic approaches.
Looks very promising. For those who do this test, how often do you do it?
As an aside, I found out today that if you are a man and you take a pregnancy test and it comes back positive, you probably have testicular cancer. It’s a quick test for a type of cancer.
You do not want this baby.
Has anyone taken the Galleri screen? I thought it might be a reasonable decision point for whether to advance to the MRI screen. Attia is down on the latter, not in principle but because of the demonstrated variance introduced by positioning, technician skills, and low test/retest reliability. Analogous points can be made when considering the Galleri test but it represents a smaller incremental step.
Thoughts.
I’ve heard, that while the blood tests are a nice to have, if only doing one thing, the MRI’s catch more things and are a better investment. If this thinking has changed, I’ll be interested to learn more about it!
Attis discusses this in a few one-off discussions inside other topics. He has considerable personal experience with them and says even slight differences in body position change results. His main point is that whereas you can compare CT-scans across the world, irrespective of who ran the machine or the brand of the machine, none of that is true of MRIs. He also emphasizes that the only way a full body MRI can usually make sense is if it is being done in collaboration with a physician who is an expert in interpreting the results back to your personal case history. Based on what little I have learned, I don’t know that it is accurate to say that one screen catches more than the other. But it does seem to be true that MRI’s “catch” eight out of every two problems, leaving you in a quandary…