No I haven’t put much attention to it yet, and I’d wait for NootropicsDepot’s TUDCA.
You could test your vitamin D levels, you might be deficient or something else.
Are they going to release TUDCA? By the way, any idea why none of the most reputable supplement manufacturers sell TUDCA? It only seems to be sold by brands I’ve never heard of, with the exception of Double Wood and Nutricost, neither of which is one of my go-to brands.
Yes, they’re working on it. I don’t know, but MYASD (CEO) have said IIRC that the real TUDCA was expensive, so they were expecting other companies to sell other bile acids disguised as TUDCA. They found a supplier for it though recently at a better price.
I don’t pretend to know what’s going on, but my wife takes quite a bit of TUDCA, so I started taking it one pill 4 times a week and my ALP went from the 100’s (like yours) to 67 this time. Just did my yearly and put the numbers in Levine and got one of the best I ever have gotten. Actual age 64, Levine age 51. I don’t know if it’s meaningless because I’m cheating on the ALP or not.
I’d rather be 51, but I’ll take it.
How much TUDCA does your wife take daily in mg? How much is each pill? Have you considered taking more? It would be interesting to see if a higher dose leads to an even lower ALP.
Ora Biomedical tested UDCA and TUDCA on worms btw: Ora Biomedical Million Molecule Challenge Results - #352 by adssx
The results don’t confirm the 2024 Chinese paper: UDCA (ursodiol) / TUDCA for healthspan and lifespan? - #4 by adssx
Ok, I may have overstated. Just asked her and she takes 2 pills when it hurts. She has done that twice a day, but not usually. But there is a twist.
I bought the body bio, which costs twice as much and has half the TUDCA. Because like I said I’m a bad shopper and I happened to be on fullscript. She says it works much better and she quit taking hers. Hers was the VINATURA, which has 1000mg plus milk thistle. She says it doesn’t work at all. Also it doesn’t smell or taste like bile, which the bodybio does. So there is a big difference in product here according to her.
WOW! wish I had come across this earlier! i.e that weight loss can catalyze? cause? gallstones.
After taking only the "trainer"dose of Rybelsus for about two weeks, had severe back pain, and ran sky high WBC. A CRT showed incipient gallstones and there may have been a small kidney stone passed.
I associated this with the Rybelsus, but now I see that it was the weight loss (went from 104 to about 97 in just a couple of weeks) might well have been the absolute causal.
If this keeps up, TUDCA will soon be a prescription -only drug. I wonder if this is why the big-name nutritional supplement companies aren’t yet manufacturing it – fear of lawsuits by big pharma(?)
Incredible! Thanks for sharing. (How did I miss this paper? ![]() )
)
A double-blind, placebo-controlled trial was completed in people with progressive MS (PMS), randomized to receive either TUDCA (2 g/day) or placebo for 16 weeks.
In the observational cohort, higher primary bile acid levels at baseline predicted slower whole-brain atrophy, brain substructure atrophy, and specific retinal layer atrophy. In the clinical trial, 47 participants were included in our analyses (21 in placebo arm, 26 in TUDCA arm). Adverse events did not differ significantly between arms (p = 0.77). The TUDCA arm demonstrated increased serum levels of multiple bile acids. No significant differences were noted in clinical or fluid biomarker outcomes. Central memory CD4+ and Th1/17 cells decreased, while CD4+ naive cells increased in the TUDCA arm compared to placebo. Changes in the composition and function of gut microbiota were also noted between the two groups.
BTW, I tried TUDCA in November and March and it caused very mild skin rash so I stopped after a couple of days. I mentioned it to @John_Hemming who said it might actually a good sign: the body clearing an infection previously ignored by the immune system. So I decided to give another try to TUDCA and third time lucky… I got a skin rash again but time it wasn’t mild it was… shingles! ![]() Any joking aside I don’t know if TUDCA caused my shingles or if John theory is correct but together with the above paper and TUDCA’s immuno-modulatory effects, that’s… Interesting.
Any joking aside I don’t know if TUDCA caused my shingles or if John theory is correct but together with the above paper and TUDCA’s immuno-modulatory effects, that’s… Interesting.
I ordered from Vitality Pro as they seem reliable + third party tested: https://vitality-pro.com/product/tudca/
How good is Nutricost as a brand?
Seems like a mixed bag after a brief perusal of various tested products on ConsumerLab. Most tested products passed, but (for instance) their goldenseal extract failed (only 1mg berberine detected per capsule instead of 30mg expected).
I’d stick with the Vitality Pro if I were you.
Numerous secreted bacterial metabolites were identified with bioactivity in various neoplasias, including ovarian cancer. One such metabolite is ursodeoxycholic acid (UDCA), a secondary bile acid that has widespread beneficial effects in neoplasias. Hereby, we assessed the bioactivity of UDCA in cell models of ovarian cancer, by applying UDCA in concentrations corresponding to the serum reference concentrations of UDCA (300 nM). UDCA induced epithelial-to-mesenchymal transition (EMT), increased the flux of glycolysis and reduced the naturally occurring oxidative stress in ovarian cancer cells. These changes were dependent on the activation of NRF2. The tumoral overexpression of UDCA-induced genes in humans correlated with worse survival. These results point out that bacterial metabolites may have opposite effects in different neoplasias and raise the possibility that UDCA-containing remedies on the long run may support cancer progression in ovarian cancer patients.
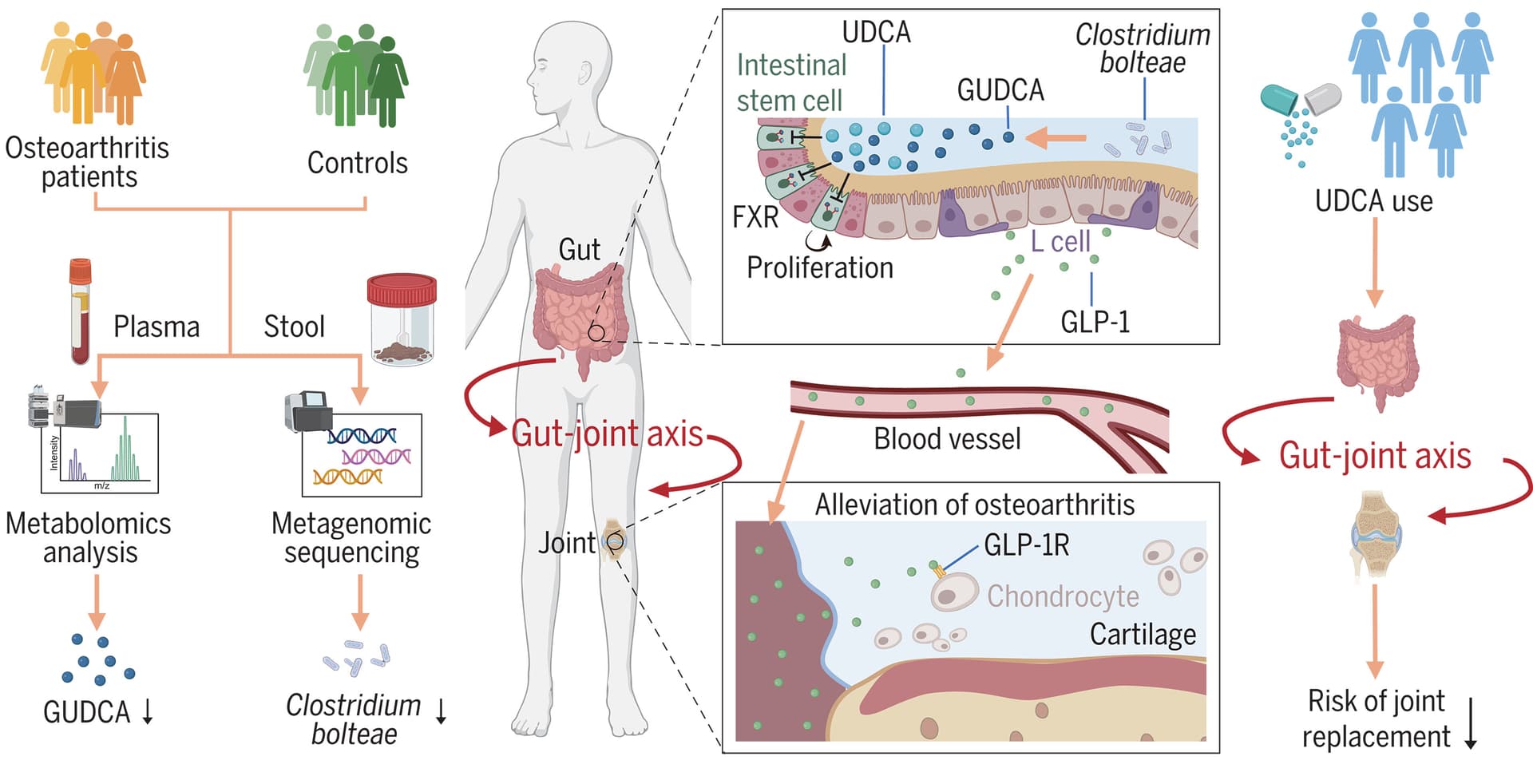
Osteoarthritis treatment via the GLP-1–mediated gut-joint axis targets intestinal FXR signaling 2025
Additionally, C. bolteae showed the strongest positive correlation with GUDCA within the same cohort. In mice, colonization with C. bolteae increased the levels of ursodeoxycholic acid (UDCA) (a precursor of GUDCA) and alleviated the progression of osteoarthritis. Notably, UDCA (an FDA-approved drug) supplementation mitigated osteoarthritis progression through this gut-joint axis in mice, and UDCA use was also associated with a lower risk of clinically relevant end point of osteoarthritis-related joint replacement in a cohort of 5972 individuals.
Interesting report on high-dose UDCA @John_Hemming:
Overlap syndromes are inflammatory rheumatic conditions in which patients have clinical manifestations suggestive of multiple distinct immune diseases. The diseases most commonly involved in overlap syndromes include rheumatoid arthritis, lupus, scleroderma, and myositis.
High-dose ursodeoxycholic acid successfully treats overlap syndrome 2025
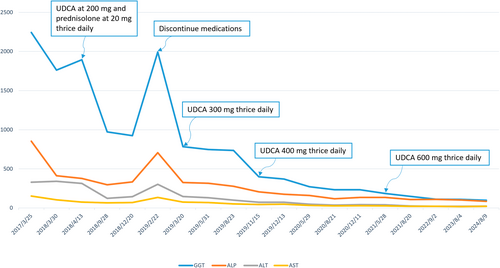
Treatment of PBC with UDCA has been shown to have a beneficial effect and highly safe effect on the disease progression. Regarding the dose of UDCA in patients with PBC, 14–16 mg/kg/day of UDCA for at least 2 years has demonstrated significant biochemical and histological improvement. Another randomized trial conducted demonstrated that UDCA doses of 13–15 mg/kg/day showed the highest rates of biochemical improvements compared with higher levels (23–25 mg/kg/day) and lower (5–7 mg/kg/day) doses. However, this patient had to take 35 mg/kg/day (700 mg thrice daily) to significantly improve the biochemical values. Therefore, UDCA can be administered at a dose that “at least does no harm”, and if this therapy fails to elicit an adequate biochemical response, a corticosteroid (e.g., prednisone 10–15 mg/kg/day) is added. However, in this case, because the patient refused to take steroids, high doses of UDCA were administered to achieve a therapeutic biochemical response. To our knowledge, administration of such high doses of UDCA to treat PBC is the first to be reported.
Also interesting (Chinese paper in cell model): Tauroursodeoxycholic Acid Induces Liver Regeneration and Alleviates Fibrosis Through GATA3 Activation 2025
Open label trial without a placebo group, but still encouraging:
Chinese paper, fairly high dose and animal model but not great for UDCA: Thyroid hormone ameliorates ursodeoxycholic acid-induced heart damage in zebrafish embryos
Ursodeoxycholic acid (UDCA) is the most commonly prescribed treatment for intrahepatic cholestasis of pregnancy (ICP) worldwide. Clinical studies show that UDCA helps alleviate maternal pruritus and lowers serum bile acid levels. However, despite treatment, women with ICP still experience unpredictable fetal deaths and other serious pregnancy complications. In this study, we hypothesize that while UDCA improves maternal symptoms, it may have harmful effects on the fetus due to its placental barrier crossing, potentially leading to negative pregnancy outcomes. We found that UDCA concentrations above 150 mg/L can exert toxic effects on zebrafish embryos, including increased mortality, slower heart rates, and pericardial edema. At these concentrations, UDCA also triggered apoptosis and elevated oxidative stress in embryonic cardiomyocytes. Importantly, the thyroid hormone T4 was able to partially mitigate these toxic effects. These findings suggest that adverse pregnancy outcomes in ICP may be related to fetal heart damage caused by UDCA’s effects on circulation, providing a possible explanation for fetal death in clinical settings. This also underscores the need to optimize UDCA dosing in clinical practice and explore the use of thyroid hormones as a potential protective therapy for the fetus in ICP cases.
TUDCA resulted in smaller lesion observed on days 1 and 7 MRIs post-TBI.
TUDCA therapy resulted in lower cortical edema on day 1 post-TBI.
Animals in TUDCA group exhibited lower level of anxiety on day 7 post-TBI.
I was under the impression that TUDCA was to be used intermittently as a liver cleanse. Maybe used a month or two in a row and then 3-4 month break would have a different result?
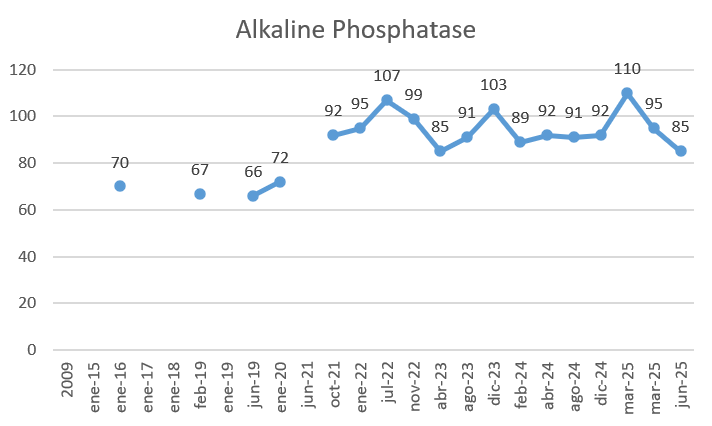
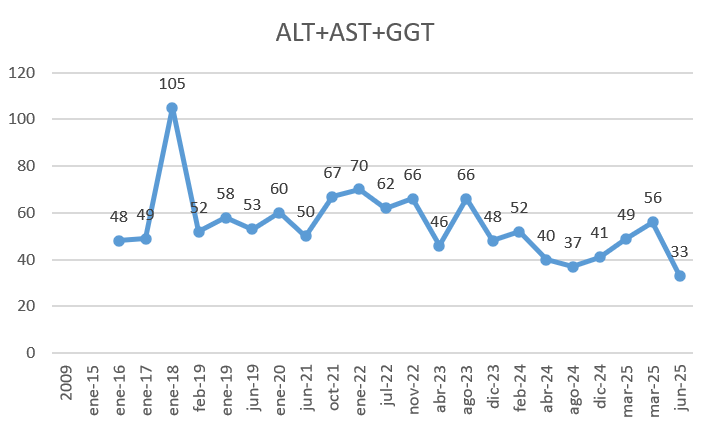
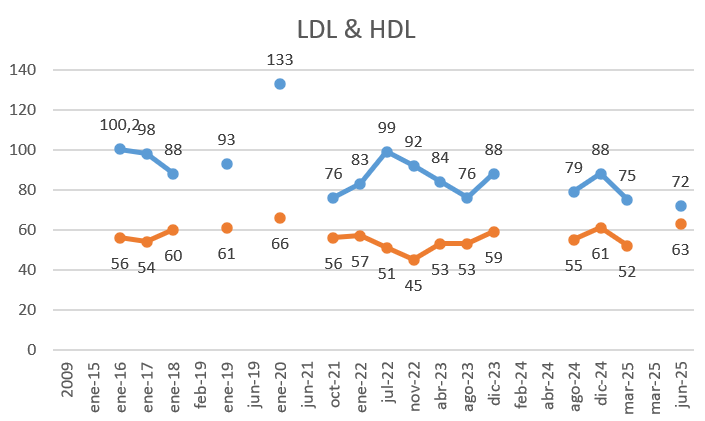
Here are my results from my TUDCA intervention (3 months, 500mg/day, normally 1h after a meal):
- My alkaline phosphatase, the objective of this intervention got a modest improvement:
- GGT+ALT+AST. my lowest data yet in all 3 biomarkers:
- LDL got my lowest reading with an improvement in HDL:
Other offtopic improvements that may be related may be not: lowest acid uric value ever & lowest homocisteine ever.
Cofounders: artichoke extract (also known to improve bile flow), increase astaxanthin from 12 to 18mg, k2 from 100 to 200mg)
Overall I would say that this has move the needle more than Rapa, 17alphaestradiol or astaxanthin, all 3 didn’t show any effect in my data. Unless someone convinces me this is doing some kind of harm I will continue. I may wait for the next test to reconfirm these results without increasing dose.
IMPORTANT: if anyone wants to try this supplement you have to know this is the most horrible tasting supplement you will ever know (and I tried all of them). You HAVE to take it in pill form.