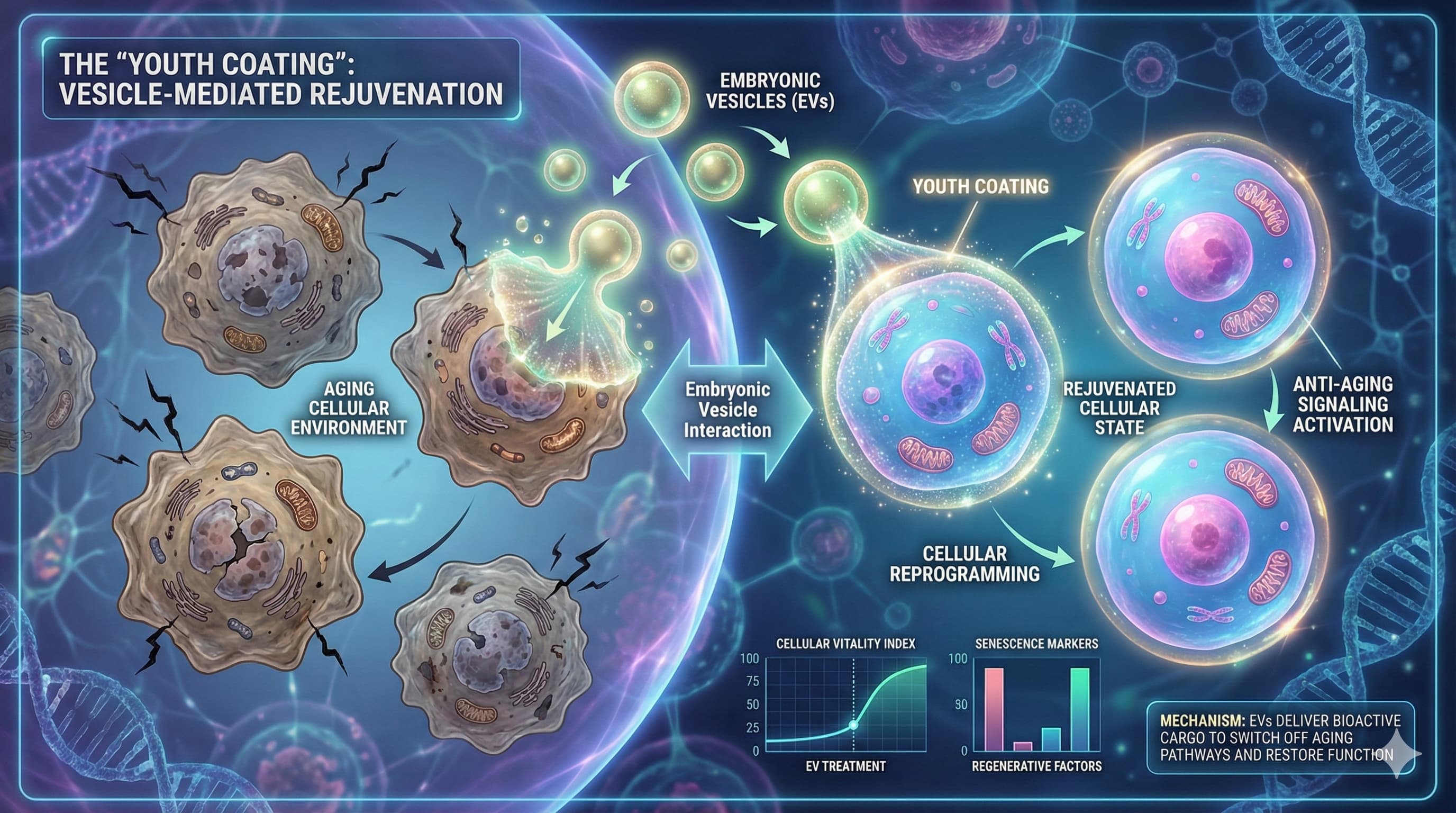
A team of researchers at Cornell University has identified a specific molecular “coating” on the surface of embryonic stem cell-derived (EVs) that appears to be the master switch for their potent anti-aging effects. While the scientific community has long known that “young” EVs can rejuvenate “old” cells, the precise mechanism has remained a “black box”—until now.
In a study published in October 2025, lead author Shun Enomoto and senior author Richard Cerione demonstrated that EVs shed by mouse embryonic stem cells (mESCs) are not merely sacks of genetic cargo, but are coated externally with the extracellular matrix protein fibronectin. This fibronectin coating is the critical “key” that unlocks the antioxidant defenses of recipient cells. Upon contact with aging fibroblasts (skin cells) and astrocytes (brain cells), the fibronectin-coated vesicles bind to integrin receptors, triggering a kinase cascade (FAK/AKT) that stabilizes the “longevity factor” Nrf2. This effectively rewires the aging cell to neutralize oxidative stress and escape senescence.
Impact Evaluation: The Journal of Biological Chemistry has an Impact Factor of approximately 3.9–4.0 (2024/2025 data). Evaluated against a typical high-end range of 0–60+ for top general science, this is a High impact journal (historically the “workhorse” of rigorous mechanistic biochemistry), though not an “Elite” broad-interest outlet like Nature or Cell. Its reputation for technical rigor validates the specific signaling pathway identified.
The Biohacker Analysis
Study Design Specifications
- Type: In Vitro (Cell Culture mechanistic study).
-
Subjects:
- Source: Mouse Embryonic Stem Cells (mESCs).
- Targets: Mouse Embryonic Fibroblasts (MEFs) and Astrocytes.
- Controls: EVs from differentiated cells (non-stem) or Fibronectin-depleted EVs.
Organ Priority: Skin (Fibroblasts) and Brain (Astrocytes).
Novelty
The “Dogma Shift” here is the focus on the corona (surface protein coating) rather than the lumen (internal cargo). Most exosome startups are obsessed with what is inside the vesicle (miRNAs). This paper suggests that the mere contact of a fibronectin-coated vesicle with a cell is sufficient to trigger the Nrf2 longevity response, even before the cargo is unloaded.
Critical Limitations
- No In Vivo Data: The study does not show that injecting these EVs into old mice extends life. It only shows that it keeps cells “young” in a dish. This is a massive translational gap.
- Tumorigenicity Risk: Embryonic stem cells (ESCs) are associated with teratoma formation. While EVs are cell-free, the growth-promoting signals (AKT activation) they carry are pro-proliferative. Activating AKT and Nrf2 in a pre-cancerous cell could theoretically accelerate tumor growth.
- Species Gap: Verified in mouse cells. Human validation is the required next step.
Actionable Intelligence
The Translational Protocol
Warning: This study uses a complex biologic (EVs), not a simple drug. The following extrapolations apply to “Exosome Therapy” and “Nrf2 Activators.”
-
Human Equivalent Dose (HED) for Exosome Therapy:
- Based on standard mouse-to-human EV scaling (typically 100 µg protein/mouse):
- Math: 100 µg / 0.02 kg mouse = 5 mg/kg mouse dose.
- HED: $5 \text{ mg/kg} \times (3/37) \approx 0.4 \text{ mg/kg}$.
- For a 70kg Human: ~28 mg of purified Exosome protein (intravenous).
- Note: Commercial “exosome” clinics often under-dose significantly compared to this academic standard.
-
Pharmacokinetics (PK/PD):
- Bioavailability: EVs injected IV have a half-life of 2–30 minutes in circulation before being cleared by the liver/spleen/lungs. However, their signaling effects (like Nrf2 activation) can last for hours to days.
- Targeting: Fibronectin-coated EVs may naturally home to injured tissues expressing specific integrins.
-
Safety & Toxicity Check:
- NOAEL: Not established for Embryonic EVs in humans.
- Red Flag: AKT Activation. Chronic activation of AKT is a known driver in many cancers. “Pulsing” this therapy is safer than chronic elevation.
- Immunogenicity: Allogenic (donor-derived) EVs can trigger immune responses, though less than whole cells.
Biomarker Verification Panel
-
Efficacy Markers:
- Nuclear Nrf2: Hard to measure clinically.
- Downstream Targets: Increase in GSH (Glutathione) levels and HO-1 (Heme Oxygenase-1) protein in PBMCs (blood cells).
- Senescence Load: Reduction in p16INK4a expression in T-cells.
-
Safety Monitoring:
- Cancer Screen: Because this pathway (AKT/Nrf2) protects cells from dying, it can also protect cancer cells. Strict annual cancer screening (Liquid Biopsy/Galleri test) is mandatory if using potent Nrf2 activators or growth factors.
Feasibility & ROI
- Sourcing: Not Available. You cannot buy “Fibronectin-coated Embryonic EVs” legally for human use.
-
The “Hack”: The study identifies Nrf2 as the goal.
- Alternative: Sulforaphane (Broccoli sprout extract) is a validated dietary Nrf2 stabilizer, though it works via Keap1 inhibition, not the FAK/AKT axis.
- Alternative: Astaxanthin helps preserve mitochondrial function and may crosstalk with this pathway.
- Cost: High-end “Exosome Therapy” in offshore clinics costs $5,000–$15,000 per session. The ROI is Low until human efficacy is proven; the risk-to-reward ratio is poor compared to standard small molecules.
Population Applicability
- Contraindications: Active Cancer. Do not activate Nrf2 or AKT if you have a tumor; it protects the tumor from oxidative stress (chemo/radiation resistance).
The Strategic FAQ
-
Q: If I can’t get Embryonic EVs, can I just take Fibronectin supplements?
- A: No. Fibronectin is a massive protein that would be digested in the gut. Even if injected, it needs to be presented on a membrane surface (like an EV) to cluster integrins correctly to fire the FAK signaling signal. Free-floating fibronectin does not have the same effect.
-
Q: Does this confirm that “Young Blood” (Parabiosis) works via exosomes?
- A: It strengthens the case. This paper aligns with previous work (e.g., Nature Aging 2024) showing young plasma EVs extend life. It adds the specific mechanism (Fibronectin/Nrf2) that was previously vague.
-
Q: Is Nrf2 activation always good?
- A: No. “Nrf2 addiction” is a phenomenon in cancer cells where they use Nrf2 to survive chemotherapy. You want cyclic activation (pulsed) to clear senescent cells or repair damage, not chronic activation which might promote malignancy.
-
Q: How does this compare to Rapamycin (mTOR inhibition)?
- A: They are opposite but complementary. Rapamycin slows growth and induces autophagy (cleaning mode) by inhibiting mTOR. These EVs activate AKT (growth mode) and Nrf2 (defense mode). You wouldn’t want to do both simultaneously; they might cancel each other out. A cycling protocol (Growth vs. Repair) would be the theoretical approach.
-
Q: Are commercial “Exosome” IVs in clinics the same as this?
- A: No. Commercial exosomes usually come from Mesenchymal Stem Cells (MSCs) from umbilical cords or fat. This study used Embryonic Stem Cells (ESCs). The protein coating and cargo are likely different. We do not know if MSC-exosomes use this specific Fibronectin-Nrf2 pathway.
-
Q: What is the “half-life” of the anti-aging effect?
- A: In the dish, the effect “potently blocked” senescence during the stress window. In vivo, because EVs are cleared in minutes, the signaling ripple effect likely lasts 24–48 hours. This suggests a need for frequent dosing, which makes it financially unfeasible for most.
-
Q: Can I boost my own endogenous stem cell EVs?
- A: Likely yes, via exercise and fasting, which mobilize stem cells. However, as you age, your stem cells (and their EVs) degrade. The paper implies you need embryonic (pristine) EVs to get the full “reset.”
-
Q: Did they check for teratomas (tumors) in this study?
- A: Data Absent. The study was in vitro (cell culture). Teratoma risk is a primary concern with any embryonic-derived product and must be ruled out in future mouse studies.
-
Q: Is there a small molecule that mimics this FAK-AKT-Nrf2 axis?
- A: There are FAK inhibitors (for cancer), but few specific FAK activators. This highlights why biologics (EVs) can do things small molecules can’t—they can structurally cluster receptors to turn on a complex pathway.
-
Q: What is the “Kill Switch” if this therapy goes wrong?
- A: There isn’t one. Once you inject exosomes, they distribute systemically. This is why safety trials for ESC-derived products are incredibly slow and rigorous.
Source Paper (Open Access): Embryonic stem cell-derived extracellular vesicles delay cellular senescence by inhibiting oxidative stress
Related Reading: Forever young? Extracellular vesicles may be key to halt aging | Cornell Chronicle