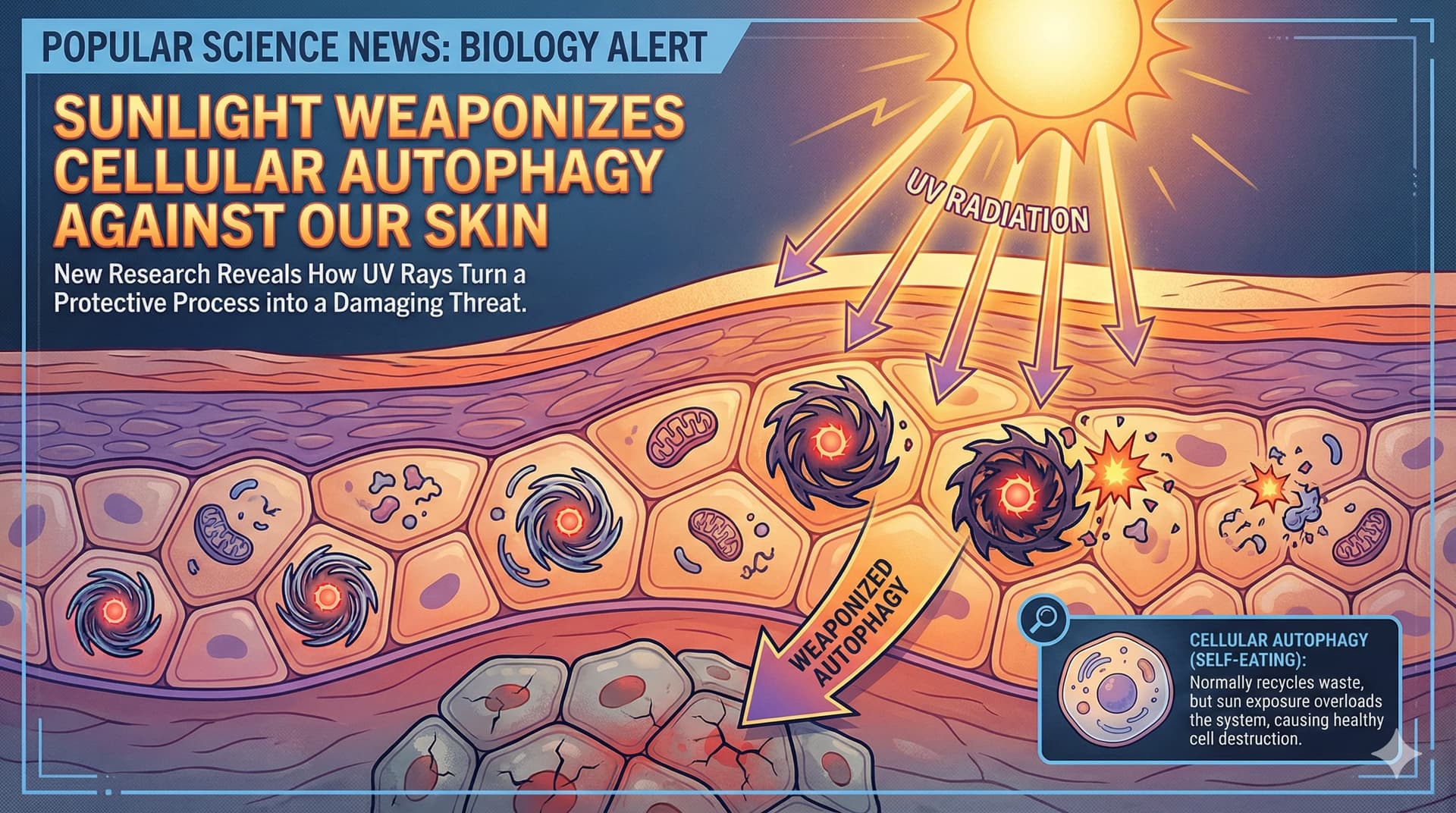
In a paradigm-shifting study published in Nature Communications, researchers from the University of Chicago have exposed a treacherous flaw in the biological machinery of human skin—a mechanism where the body’s own cleaning systems are turned against it by sunlight. The research, titled “YTHDF2 regulates self non-coding RNA metabolism to control inflammation and tumorigenesis,” unravels a molecular tragedy that plays out in our cells every time they are exposed to ultraviolet B (UVB) radiation.
At the center of this drama is a protein called YTHDF2, a “reader” of the epigenetic code that normally acts as a guardian of cellular identity. Its job is to identify and destroy fragments of “self” RNA—specifically a molecule called U6 small nuclear RNA (snRNA)—that have been chemically modified with a marker known as N6-methyladenosine (m6A). Without YTHDF2, these RNA fragments accumulate and drift into the cell’s waste-processing centers, the endosomes. There, in a case of mistaken identity, the immune sensor Toll-like Receptor 3 (TLR3) confuses these harmless self-RNAs for viral invaders.
The University of Chicago team, led by Dr. Yu-Ying He and Dr. Chuan He, discovered that UVB radiation triggers a “kill switch” for YTHDF2. In a cruel twist, the radiation activates autophagy—the very process usually praised for extending life and cleaning cells—to specifically target and digest the YTHDF2 protein. With the guardian removed, the “viral mimic” U6 snRNA floods the system, triggering a massive inflammatory response that drives sunburn, tissue damage, and eventually, skin cancer.
This discovery rewrites the rulebook for longevity and dermatology. It suggests that the chronic inflammation of aging, or “inflammaging,” is not merely a passive accumulation of damage, but an active, runaway immune response triggered by a failure in RNA quality control. By identifying the specific molecular pathway—from the m6A tag to the lysosomal transporter SIDT2—the authors have illuminated new targets for intervention. If we can stabilize YTHDF2 or block the U6-TLR3 handshake, we might finally decouple the sun’s rays from the aging they cause, creating a biological “sunblock” that works from the inside out.
Actionable Insights for N=1 Experimentation
Disclaimer: These insights are theoretical and based on the reviewed literature. They do not constitute medical advice.
4.1 Biomarkers to Watch
To track the efficacy of interventions targeting the YTHDF2-TLR3 axis:
- hs-CRP (High-sensitivity C-reactive Protein): The gold standard for systemic inflammation.
- IL-6: A specific cytokine downstream of the TLR3-NF-κB pathway.
- Erythema Response Time: A functional metric. Measure the time it takes for skin to redden under a constant UV source (e.g., natural noon sun). Increased resistance suggests maintained YTHDF2 integrity.
- Ferritin: Often elevated in acute inflammation and macrophage activation.
4.2 The “Solar Shield” Protocol (Stacking)
Phase 1: Prevention (The “Armor”)
- Topical Zinc Oxide: Non-negotiable. Blocks the UVB trigger.
- Oral Nicotinamide (500mg BID): Proven to reduce new non-melanoma skin cancers and support DNA repair (PARP activity), acting synergistically with RNA protection.
Phase 2: Downstream Blockade (The “Dampener”)
- Resveratrol (500mg) + Curcumin (500mg Phytosome): Both are potent inhibitors of NF-κB. They act as a firewall, preventing the signal from the endosome (TLR3) from reaching the nucleus.
- Quercetin (400mg): Acts as a mild endosomal modulator and zinc ionophore, potentially altering the pH environment required for TLR3 signaling.
Phase 3: The Methylation Support (The “Tagging”)
- TMG (Trimethylglycine) or Choline: Ensures ample supply of S-adenosylmethionine (SAMe), the methyl donor required by METTL3 to tag U6 snRNA with m6A. Without the tag, YTHDF2 cannot recognize and degrade the debris.
Phase 4: Autophagy Timing (The “Timer”) (Be Careful with Sun Exposure when dosing rapamycin)
- Pulsed Rapamycin: If using rapamycin, take it in the evening or on days of low sun exposure. Taking a potent autophagy activator immediately before beach exposure is theoretically contraindicated by this study, as it could accelerate YTHDF2 loss.
4.3 Dosing Strategy
- Circadian Alignment: The study implies YTHDF2 levels fluctuate. Support circadian biology (sleep, dark exposure) to maximize natural protein synthesis and repair cycles.
- “Vacation Protocol”: During periods of intense UV exposure (vacations), discontinue autophagy activators (fasting, rapamycin) and switch to “building” (mTOR activation via protein) and “shielding” (antioxidants) to preserve protein mass, including YTHDF2.
Key Metrics:
- Journal Rank: Nature Communications (Impact Factor ~16.6; Top-tier multidisciplinary journal).2
- Authors’ Affiliation: University of Chicago, USA.2
- Core Mechanism: UVB > YTHDF2 Dephosphorylation > Autophagic Decay > U6 snRNA Accumulation > Endosomal TLR3 Activation > Inflammation/Tumorigenesis.
Read Gemini’s full research paper analysis: https://gemini.google.com/share/b653054eb822
Source Paper (Open Access): YTHDF2 regulates self non-coding RNA metabolism to control inflammation and tumorigenesis (Nature Communications)