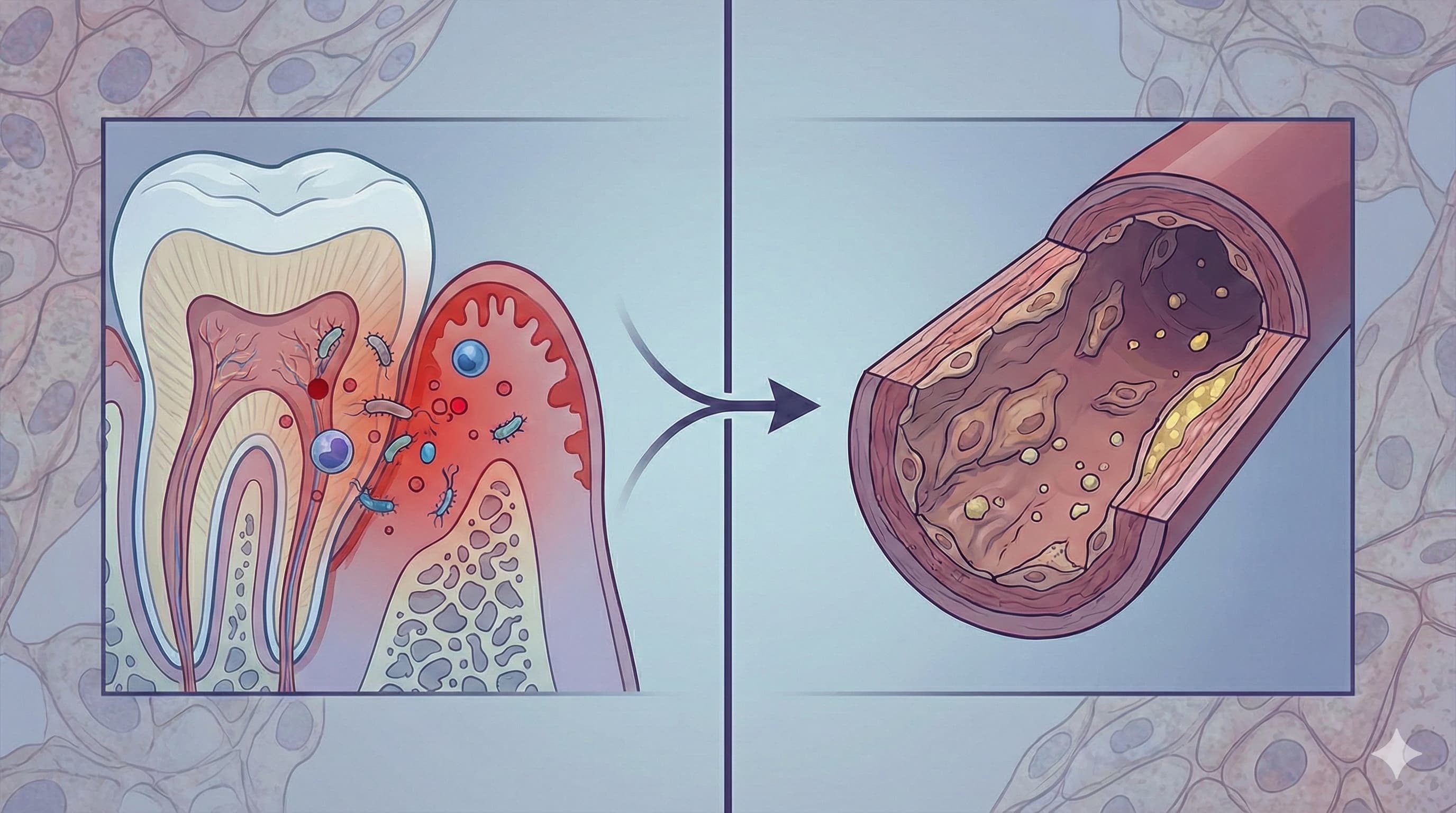
Forget the simplistic “floss or die” mantras of the 90s. This newly published review synthesizes a decade of mechanistic data to validate a specific, causal pathway: Periodontal disease is not just a marker of aging; it is an active driver of vascular senescence. The authors rigorously demonstrate that the chronic inflammatory burden from the oral cavity (specifically Porphyromonas gingivalis infection) directly impairs Flow-Mediated Dilation (FMD)—the gold-standard metric of how well your arteries can dilate.
The critical finding for biohackers is the reversibility demonstrated in the cited clinical trials. Aggressive periodontal therapy doesn’t just “save teeth”; it restores endothelial nitric oxide (NO) production and lowers systemic arterial stiffness, effectively turning back the vascular clock. However, the data reveals a “healing crisis”: intensive treatment causes a massive, acute spike in systemic inflammation (endothelial shock) before the long-term benefits kick in—a detail often omitted in public health messaging.
Open Access Research paper: Vascular Endothelial Function in Periodontal Disease: Role of Inflammation
Institution: Hiroshima University, Japan Journal: Journal of the American Heart Association (JAHA) Impact Evaluation: The impact score of this journal is ~5.3 (Impact Factor) therefore this is a High impact specialty journal (Cardiology).
Part 2: The Biohacker Analysis
Study Design
- Type: Systematic Review & Meta-Synthesis of Clinical Intervention Trials.
- Subjects: Humans (Aggregating data from healthy subjects, CAD patients, and Type 2 Diabetics across >16 major studies, including the landmark Tonetti et al. NEJM trial).
-
Lifespan Data:
- Mortality Risk: Associated with a 22% to 59% increased risk of all-cause and cardiovascular mortality (based on cited epidemiological data).
- Healthspan Proxy: Reversal of endothelial dysfunction (FMD improvement) is a surrogate for delaying atherosclerotic onset by estimated 5–10 years.
Mechanistic Deep Dive
The paper moves beyond “inflammation” to identify specific molecular assassins:
- NO Uncoupling: Chronic exposure to oral bacterial endotoxins (LPS) downregulates eNOS (endothelial nitric oxide synthase), turning the vessel wall into a rigid pipe.
- ADMA Elevation: Periodontitis elevates Asymmetric Dimethylarginine (ADMA), an endogenous inhibitor of NO production. High ADMA is a potent predictor of cardiovascular death.
- The “Leaky Gum” Pathway: Ulcerated pocket epithelium allows daily translocation of bacteria into the bloodstream (bacteremia), triggering a hepatic acute-phase response (CRP spikes) that degrades the endothelial glycocalyx.
Novelty & “What We Didn’t Know Yesterday”
While the link is old, this review solidifies the causality of reversibility. Previous consensus was skepticism on whether treating gums actually fixed the heart. This paper confirms that Intensive Periodontal Treatment (IPT) yields a measurable improvement in vascular function (FMD) after 6 months, verifying that the damage is not permanent scarring but a functional suppression that can be lifted.
Critical Limitations
- The “Acute Shock” Hazard: The review highlights that endothelial function actually worsens 24 hours post-treatment due to bacteremia from the cleaning itself. This is a critical safety signal often ignored.
- Heterogeneity of Protocols: The “treatment” varies wildly across studies—from simple cleaning to surgery with antibiotics. There is no standardized “Longevity Dental Protocol.”
- Lack of Hard Outcomes: While FMD improves, we still lack a massive randomized trial proving that treating gums reduces heart attacks (hard endpoints) directly. We are relying on a surrogate marker (endothelial function).
- Reverse Causality Risk: [Confidence: Medium] It remains possible that people with better endothelial function simply heal their gums better, creating a chicken-and-egg confounder.