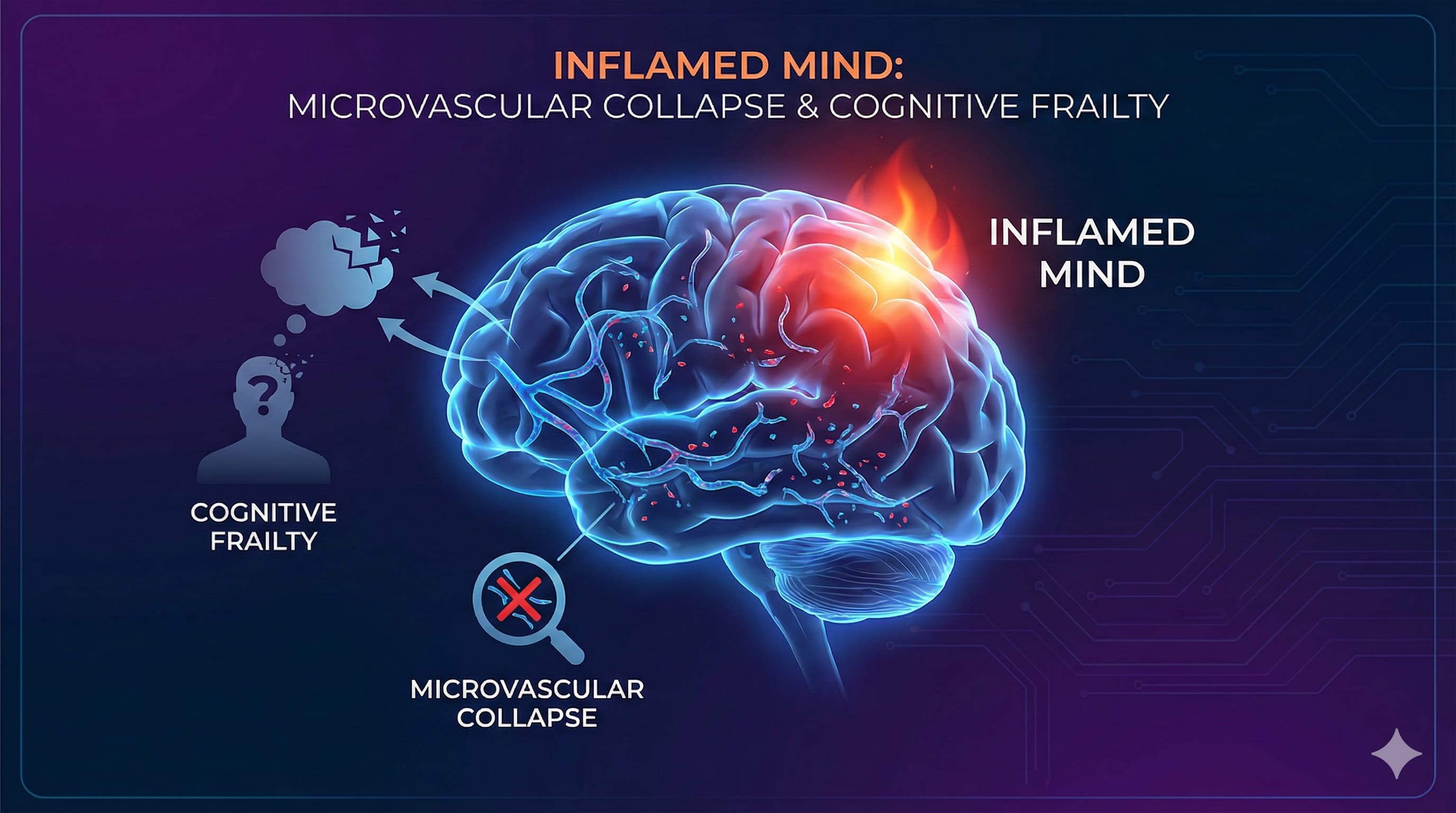
The Silent Burn: How Systemic Inflammation Dismantles the Brain’s Hydraulic Infrastructure
In a paradigm-shifting review published in Experimental Gerontology , researchers from China Medical University have re-framed the etiology of “Cognitive Frailty”—the dangerous intersection of physical weakness and cognitive decline. Moving beyond the neuron-centric models that have dominated Alzheimer’s research for decades, the authors propose a “vascular-inflammatory axis” as the primary driver of this condition. The central thesis is stark: the aging brain does not simply wither; it starves, choked off by a collapsing microvascular network that has been degraded by chronic, systemic inflammation.
The paper, titled “Chronic inflammation and cognitive frailty in older adults: A narrative review” (Jiang et al., 2025), synthesizes emerging evidence that pro-inflammatory cytokines—specifically IL-6, TNF-α, and CRP—are not merely passive markers of aging but active agents of structural destruction. These molecules, originating from senescent cells and visceral fat, launch a relentless assault on the body’s microcirculation, the delicate web of capillaries responsible for nourishing tissues. In the brain, this results in the breakdown of the Blood-Brain Barrier (BBB), neurovascular uncoupling, and eventually, the simultaneous manifestation of sarcopenia (muscle loss) and cognitive impairment.
For the longevity community and biohackers, this report serves as a critical biological signal: preserving cognitive function is isomorphic with preserving endothelial integrity. The authors detail mechanistic pathways, including the cGAS-STING axis—a cellular alarm system triggered by leaking mitochondrial DNA—that drives this sterile inflammation. Crucially, the review posits that this state is reversible. Interventions ranging from the Dietary Inflammatory Index (DII) optimization to specific mind-body exercises like Baduanjin are highlighted as potent levers to dampen this inflammatory fire and restore microvascular perfusion. The implication is profound: to save the neuron, one must first save the vessel.
The Sources of Sterile Inflammation
Jiang et al. identify several upstream sources of the cytokines driving CF. These sources are critical targets for biohacker interventions because they represent the “root causes” of the downstream damage.
- Cellular Senescence (The SASP): As cells accumulate DNA damage or reach their replicative limit (Hayflick limit), they enter a zombie-like state called senescence. They do not die, but they stop dividing. Crucially, they become metabolically hyperactive, secreting a toxic cocktail of pro-inflammatory cytokines (IL-6, IL-1β, IL-8), chemokines, and proteases. This is the Senescence-Associated Secretory Phenotype (SASP). In CF, endothelial cells lining the blood vessels become senescent, turning the vasculature itself into a source of inflammation.
- Visceral Adipose Tissue: Fat is not merely energy storage; it is an endocrine organ. In aging and metabolic syndrome, visceral fat becomes infiltrated with macrophages (M1 phenotype) that secrete high levels of TNF-α and IL-6. This “adipose inflammation” spills over into the systemic circulation, reaching the brain and muscle tissues.
- Gut Dysbiosis (“Leaky Gut”): The review and supporting literature point to the gut-brain axis. With age, the intestinal barrier permeability increases, allowing bacterial endotoxins (Lipopolysaccharides, LPS) to enter the bloodstream. This condition, metabolic endotoxemia, triggers a systemic immune response, chronically elevating CRP levels and driving neuroinflammation.
- Immunosenescence: The aging immune system loses its ability to distinguish “self” from “non-self” and to clear pathogens effectively. This results in a dual failure: a weakened response to new infections (like flu) but a heightened, non-specific autoimmune-like activity against the body’s own tissues.
The Neural Consequences
When these cytokines cross the Blood-Brain Barrier (or damage it sufficiently to enter), they activate the brain’s resident immune cells: the microglia. In a healthy brain, microglia are housekeepers, pruning synapses and clearing debris. In an inflamed brain, they adopt a “primed” or aggressive phenotype. They stop cleaning and start attacking, releasing reactive oxygen species (ROS) and excitotoxins (like glutamate) that kill neurons. This “neuroinflammation” is the biological bridge linking systemic inflammation to the cognitive deficits seen in CF.
Continue reading the full story in the next post, here: Interventions and Protocols: Actionable Insights
Source Paper (Open Access): Chronic inflammation and cognitive frailty in older adults: A narrative review
Gemini Analysis of the paper: https://gemini.google.com/share/11560ad80357