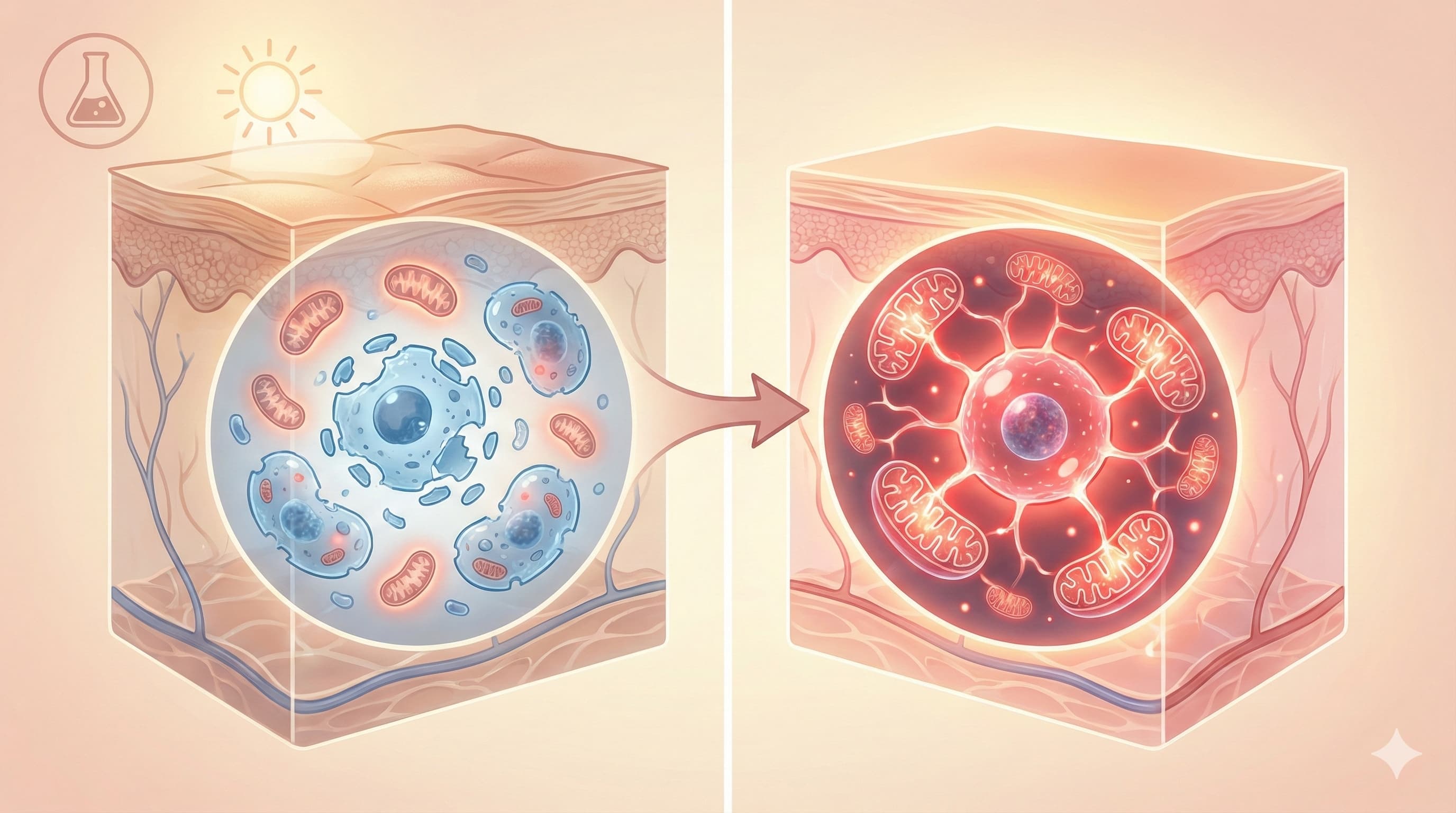
In a counterintuitive twist, researchers have demonstrated that “fighting fire with fire” is the key to reversing skin aging. While Ultraviolet Radiation (UVR) accelerates aging by generating destructive Reactive Oxygen Species (ROS), this study reveals that a precise, low-dose “flash” of ROS generated by Photodynamic Therapy (PDT) triggers a profound rejuvenation effect.
The mechanism is mitohormesis—a biological “controlled burn.” By using 5-aminolevulinic acid (ALA) and red light to induce a mild, temporary stress on mitochondria, the treatment forces skin cells to activate powerful survival and repair programs (ISR/UPR). The critical discovery here is metabolic: this hormetic stress actively clears Citrate (CA), a metabolite that was found to accumulate in and drive cellular senescence. By unclogging this metabolic jam, the treatment effectively “de-ages” the skin, restoring collagen structure, reducing wrinkles, and clearing senescent “zombie” cells without killing healthy tissue. This establishes a direct link between mitochondrial metabolism and the structural reversal of photoaging, validating a targeted, low-toxicity intervention for longevity enthusiasts.
Source:
- Date: December 31, 2025
-
Open Access Paper: Multi-Omics Analysis Reveals Photodynamic Therapy Ameliorating Skin Photoaging by Improving Cellular Senescence Through Mitohormesis-Mediated Reduction of Citrate Content
Institution: Shanghai Skin Disease Hospital, Tongji University School of Medicine, China
Journal: Aging Cell Impact Evaluation: The impact score of Aging Cell is ~7.2–8.0 (2024/2025 JIF). Therefore, this is a High Impact journal (Top 10% in Geriatrics & Cell Biology).
The Biohacker Analysis
Study Design Specifications
- Type: In vivo (Murine) and In vitro (Human cell lines).
-
Subjects:
- In vivo: Female SKH-1 hairless mice (immunocompetent photoaging model), aged 6-8 weeks.
- In vitro: Human Dermal Fibroblasts (HDF), HaCaT (keratinocytes), and Normal Human Epidermal Keratinocytes (NHEK).
- Lifespan Data: Not Applicable. This is a healthspan and tissue rejuvenation study. No maximum lifespan data was recorded.
-
Key Dosing:
- In vivo: 3% ALA cream + Red Light (633nm) at 6.0 J/cm2 (implied by 100mW/cm² for ~60s).
- In vitro: Low dose (0.5−2 J/cm2) was rejuvenating; High dose (4 J/cm2) exacerbated senescence.
Mechanistic Deep Dive
The study redefines PDT not just as a “cell killer” (used in cancer) but as a “metabolic reset switch.”
- Mitohormesis Trigger: ALA-PDT causes a transient spike in mitochondrial ROS. This mild damage does not kill the cell but activates the Integrated Stress Response (ISR) and Mitochondrial Unfolded Protein Response (UPRmt).
- The Citrate Bottleneck: The authors identified that UV-induced senescence leads to an accumulation of Citratevia the activation of the Reverse TCA cycle (rTCA) and enzyme IDH2. High intracellular citrate acts as a pro-aging signal.
- The Reset: The hormetic response to PDT downregulates IDH2 and restores forward TCA flux, effectively “draining” the excess citrate.
- Senolysis vs. Senomorphics: Interestingly, the low-dose treatment acted as a senomorphic (suppressing the SASP and reverting the senescent phenotype) rather than a pure senolytic (killing the cell), though markers of senescence (p16, p21) significantly dropped.
Novelty
- Metabolic Specificity: Identifying Citrate accumulation as a driver of skin senescence is a novel metabolic target.
- Hormetic Dosing Window: Defining the precise “Goldilocks” zone (0.5−1.0 J/cm2) where ROS switches from damaging to rejuvenating.
- Pathway Mapping: Explicitly linking the p-eif2s1 pathway (ISR) to the clearance of senescent phenotypes via PDT.
Critical Limitations
- Mouse Skin vs. Human Skin: SKH-1 mice are a standard model, but their skin mechanics and immune responses differ from humans. The in vitro human data helps bridge this, but clinical verification of the “Citrate Mechanism” is needed.
- Single Tissue Focus: The study only examined skin. It is unknown if this “Citrate Reset” applies to other organs or if systemic PDT (e.g., blood irradiation) would have similar effects.
- Duration: The study followed mice for 1 month post-treatment. Long-term safety regarding potential DNA damage from the ROS spike (even if low) remains a theoretical risk, though not observed here.
- Data Gap: No functional assay was done to see if the “rejuvenated” fibroblasts produced “younger” elastin, only collagen structure was visually assessed.
Actionable Intelligence
The Translational Protocol
- Compound: 5-Aminolevulinic Acid (ALA) (Topical).
- Commercial Availability: Available as prescription (Levulan Kerastick, Ameluz) or research-grade cosmetic ingredients.
-
Clinical Equivalent Protocol:
- Preparation: Topical application of 3% to 10% ALA cream/gel (Lower than the standard 20% used for cancer/AK to avoid excessive damage).
- Incubation: 1–3 hours in the dark (occluded) to allow conversion to Protoporphyrin IX (PpIX).
- Activation (The Critical Step): Red LED Light (630−635 nm).
-
Fluence (Dose): 3.0−6.0 J/cm2.
- Calculation: If using a standard high-power LED panel (e.g., 100 mW/cm2), the exposure time is 30 to 60 seconds.
- Warning: Standard clinical PDT often uses 10−37 J/cm2. This study suggests LESS IS MORE for rejuvenation.
- Frequency: The mice were treated weekly. For humans, a monthly or quarterly “maintenance” protocol is more realistic for anti-aging.
Biomarker Verification Panel
-
Efficacy Markers:
- Visual: Reduction in fine lines and improvement in skin hydration (TEWL).
- Biochemical (Biopsy required): Reduced p16 and p21 expression; Reduced MMP-1 (Collagenase).
- Serum (Experimental): Potential reduction in circulating SASP factors (IL-6, TNF-alpha) if treating large surface areas.
-
Safety Monitoring:
- Visual: Erythema (Redness) and Edema (Swelling) should be transient (<48h).
- Contraindication Check: Porphyria profiles (urine porphyrins) if history exists.
Feasibility & ROI
- Cost: High if clinical (Levulan stick ~$300-500 + Procedure fee). Low if DIY (ALA powder + Red Light Panel), though DIY carries significant burn risks.
- ROI: High. This is one of the few interventions with visually verifiable results that remodels the extracellular matrix rather than just moisturizing it.
Population Applicability
-
Contraindications:
- Porphyria: Absolute contraindication.
- Photosensitive Drugs: Tetracyclines, Thiazides, St. John’s Wort.
- Autoimmune Conditions: Caution advised (PDT stimulates local immune response).
- Active Melanoma: Do not use on suspicious lesions without biopsy.