Part 3: Claims & Verification
| Claim |
Verification Status |
Evidence Level |
Hierarchy Notes |
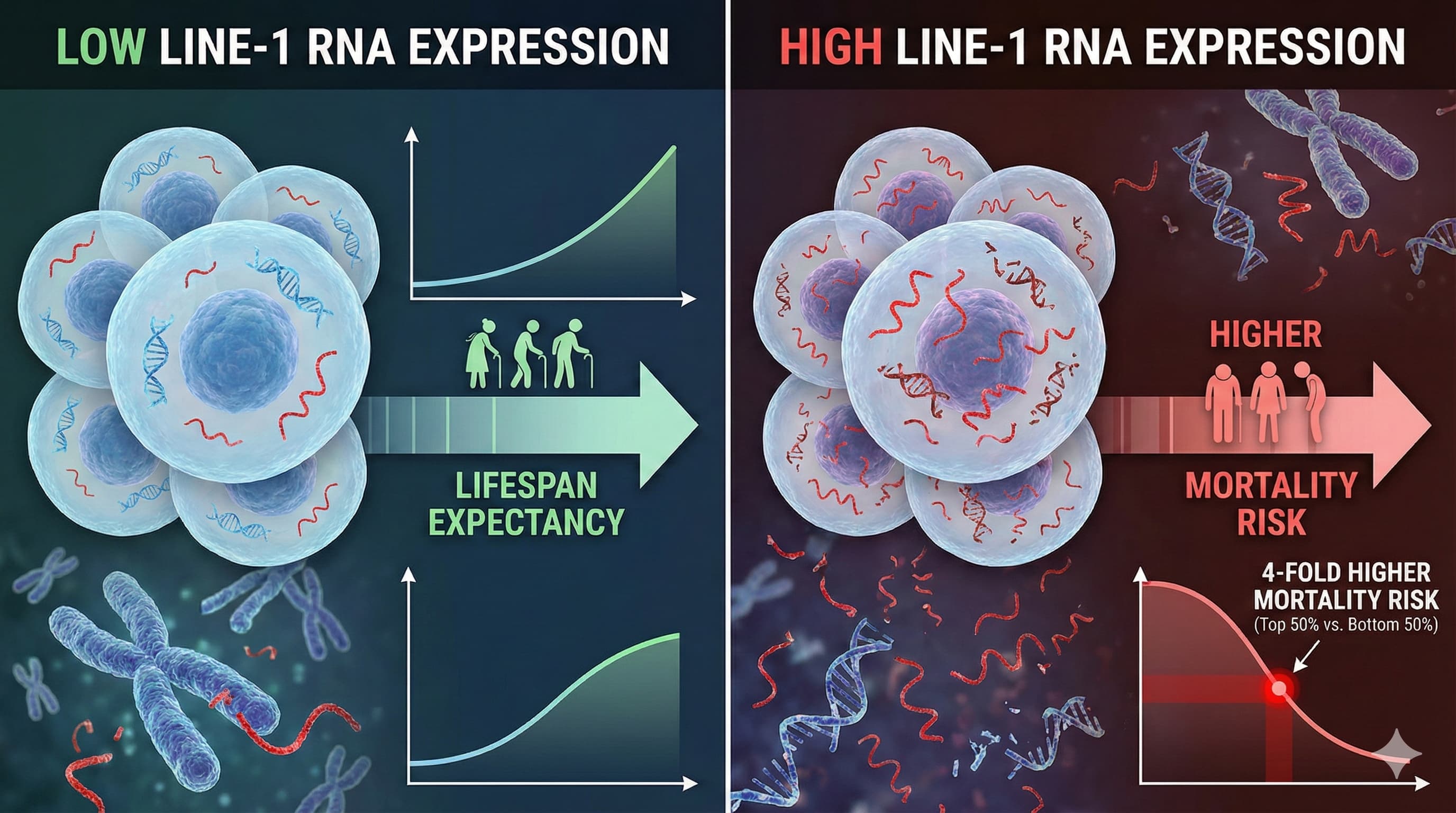
| “High LINE-1 RNA predicts human mortality.” |
Verified (in this study). Supported by cancer literature (e.g., Frontiers in Cell Dev Biology). |
Level C |
Observational correlation. High confidence in biomarker utility, lower in causality. |
| “Melatonin suppresses LINE-1 expression.” |
Verified (Belancio et al., Nucleic Acids Res, 2014). |
Level D |
Mechanistic/In vitro data. Strong logic, but lacks human clinical trials for this specific outcome. |
| “Physical Exercise reduces LINE-1 RNA.” |
Verified (Romero et al., 2019; ResearchGate reviews). |
Level C/D |
Human muscle biopsy data exists showing acute reduction. |
| “L1 Retrotransposons drive inflammation (cGAS-STING).” |
Verified (Simon et al., Nature, 2019; De Cecco et al., Nature, 2019). |
Level D |
Established mechanism in mice (SIRT6 KO) and cell models. Translational Gap to humans is small but present. |
| “L1 ASOs extend lifespan.” |
Verified (Della Valle et al., 2022). |
Level D |
Translational Gap: Only proven in Progeria mice so far. |
Safety Check:
-
Melatonin: High safety profile.
-
LINE-1 Inhibitors (NRTIs like Lamivudine): Safety Data Absent in this paper, but known from HIV literature. Risk: Lactic acidosis, pancreatitis, mitochondrial toxicity.
Part 4: Actionable Intelligence (Deep Retrieval Mode)
The study implies a protocol to “Recage the Jumping Genes.”
1. The Pharmacological Hammer: NRTIs (Lamivudine)
Background: While the paper focuses on Melatonin, the “Biohacker” drug of choice for LINE-1 is Lamivudine (3TC), a Reverse Transcriptase Inhibitor.
-
Human Equivalent Dose (HED):
-
Mouse Data: SIRT6 KO mice and SAMP8 aging mice use ~100 mg/kg via drinking water.
-
Calculation: 100 mg/kg×(3/37)≈8.1 mg/kg.
-
70kg Human: ≈567 mg/day.
-
Standard HIV Dose: 150 mg BID (300 mg/day) or 300 mg QD.
-
Recommendation: The HIV dose (300 mg/day) is likely sufficient for enzyme inhibition without the toxicity of higher doses.
-
Safety (Critical):
-
NOAEL: Well-established in HIV.
-
Side Effects: Nausea, headache. Rare/Severe: Lactic acidosis, hepatomegaly with steatosis.
-
Contraindications: Impaired renal function (requires dose adjustment).
2. The Circadian Shield: Melatonin
-
Dose: 0.5 mg – 5 mg Nightly (Timed Release).
-
Mechanism: Melatonin activates MT1 receptors which epigenetically silence LINE-1.
-
Feasibility: High. Cheap, available, safe.
-
Biomarker: Difficult to measure LINE-1 RNA commercially. Monitor hsCRP (downstream inflammation).
3. The Lifestyle Signal: HIIT Exercise
-
Mechanism: Intense exercise modulates DNA methylation in skeletal muscle, re-silencing repetitive elements.
-
Protocol: 4x45 min sessions/week including resistance training.
Cost-Benefit Analysis
-
Lamivudine: Moderate Cost ($30-$100/mo off-label). Risk: Medium. Benefit: High (Potential to stop DNA damage at source).
-
Melatonin: Low Cost ($5/mo). Risk: Low. Benefit: Moderate (Epigenetic support).
Part 5: The Strategic FAQ
1. Does Rapamycin conflict with this protocol? Answer: No, it acts synergistically. Research (e.g., Wang et al.) indicates that Rapamycin also downregulates LINE-1 expression. Combining Rapamycin (mTOR inhibition) with Lamivudine (RT inhibition) attacks the problem from two angles: preventing the expression of the RNA and blocking the function of the protein.
2. I take Metformin. Does it help? Answer: Yes. Metformin has been shown to improve epigenetic stability and may repress LINE-1 activation via AMPK pathways and methylation changes (e.g., HOXA10 promoter data). It is a “Sentinel of the Epigenome.”
3. Is there a commercial test for LINE-1 activation? Answer: Sort of. Diagnostic labs (like TruDiagnostic) offer “DunedinPACE” and methylation clocks. Some research panels look at LINE-1 Methylation (global hypomethylation is bad). However, a direct “LINE-1 RNA in blood” test is not yet a consumer product.
4. Why use Lamivudine instead of other HIV drugs? Answer: Lamivudine (3TC) has the lowest mitochondrial toxicity profile of the older NRTIs. Drugs like AZT are too toxic for longevity use.
5. Is this effect sex-specific? Answer: The study showed a much stronger correlation in women. This may be linked to the X-chromosome (which is rich in L1 elements) or hormonal differences in melatonin/heterochromatin maintenance.
6. Can I just take Vitamin B12/Folate (Methylation support)? Answer: Theoretically yes. L1 is silenced by methylation. Methyl-donor deficiency leads to L1 activation. Ensuring adequate B12/Folate/TMG is the “Level 1” defense.
7. What is the “Translational Gap” risk? Answer: High. We know L1 kills mice. We know humans with high L1 die young. We do not yet have a human trial showing that taking Lamivudine extends human lifespan.
8. Does L1 activation cause Alzheimer’s? Answer: Likely. L1 “jumping” is rampant in the brains of Alzheimer’s patients (Lee et al., Nature 2018). The reverse-transcribed DNA damages neurons. This makes L1 inhibition a prime neuroprotective strategy.
9. Is this related to “Leaky Gut”? Answer: Indirectly. Sterile inflammation (cGAS-STING) caused by L1 can compromise barrier tissues.
Follow-on Prompt:
Identify the scientific and clinical data that suggests that Lamivudine may be helpful in health and longevity by being a LINE-1 Inhibitor
Gemin Pro Response:
Report: Lamivudine as a Geroprotector (LINE-1 Inhibitor)
Executive Summary:
Lamivudine (3TC), a widely used antiretroviral drug for HIV/Hepatitis B, has emerged as a leading candidate for “repurposing” in longevity medicine. The scientific rationale is its ability to inhibit LINE-1 (Long Interspersed Nucleotide Element-1), an ancient retrotransposon (“jumping gene”) that activates during aging, causing genomic instability and sterile inflammation.
While the mechanistic data in mice (particularly the SIRT6 knockout model) is robust—demonstrating that Lamivudine can block the “inflammaging” signal at its source—human clinical data is currently in the early/mixed phase. A successful pilot trial in Alzheimer’s supports the hypothesis, but a larger trial in ALS failed to show survival benefits.
1. The Mechanism: “Putting the Genie Back in the Bottle”
The core hypothesis is that aging involves the loss of epigenetic silencing (heterochromatin), allowing ancient viral elements in our DNA to wake up.1
-
The Target (LINE-1): LINE-1 comprises ~17% of the human genome.2 In youth, it is silenced by SIRT6 and heterochromatin.3 In aging, it escapes repression, transcribing RNA that is reverse-transcribed into cytoplasmic cDNA.
-
The Signal (cGAS-STING): This cytoplasmic cDNA is recognized by the cell as a viral invasion, triggering the cGAS-STING pathway .4 This unleashes a storm of Type I Interferons (IFN-I) and inflammatory cytokines (SASP), driving tissue aging.5
-
The Intervention (Lamivudine): As a Nucleoside Reverse Transcriptase Inhibitor (NRTI), Lamivudine blocks the conversion of LINE-1 RNA into the toxic cDNA, effectively cutting the fuse of the inflammatory bomb.
2. Pre-Clinical Evidence (Level D - Strong Signal)
Study Type: Animal Models (Mice, Drosophila)
| Study / Source |
Key Finding |
Significance |
|
SIRT6 Knockout Mice (Nature, 2019) |
SIRT6-deficient mice age rapidly and die young (progeria). They have massive LINE-1 activation. Lamivudine treatment extended their healthspan, reduced inflammation, and rescued DNA damage markers. |
**Proof of Principle:**Confirms LINE-1 is a driver of the aging phenotype, not just a bystander. |
|
Alzheimer’s & Tau Models(Brown University) |
In Tau transgenic mice and fruit flies, LINE-1 is activated. Lamivudine reduced neuroinflammation and prevented neuronal death. |
Suggests potential for neurodegenerative diseases where “jumping genes” destroy neurons. |
|
Down Syndrome Mice (Ts65Dn Model) |
Lamivudine treatment rescued cognitive deficits and normalized hippocampal memory function. |
Indicates that retrotransposon activity impairs cognitive plasticity. |
3. Clinical Evidence (Level B/C - Mixed Signal)
Study Type: Human Clinical Trials
A. The Success: Alzheimer’s Disease (MCI) Pilot
-
Source: Sullivan et al. (UT Health San Antonio), 2024.6
-
Protocol: 300 mg/day (Standard Oral Dose) for 6 months.
-
Subjects: 12 patients with Mild Cognitive Impairment (MCI).7
-
Results:
-
Safety: Well-tolerated; drug penetrated the Blood-Brain Barrier (CSF).8
-
Biomarkers: Significant reduction in neurodegeneration markers (GFAP , Flt1 ) and inflammation (IL-15 ).9
-
Cognition: Participants remained stable (did not decline), though the study was too small/short to prove cognitive efficacy.
-
Takeaway: This is the strongest human signal to date that Lamivudine engages the target in the human brain.
B. The Failure: ALS (Lighthouse II Trial)
-
Source: Lighthouse II Trial (Australia/Europe).
-
Protocol: Triumeq (Abacavir/Dolutegravir/Lamivudine ).10
-
Subjects: Patients with Amyotrophic Lateral Sclerosis (ALS).
-
Results: Trial Terminated Early. The Data Safety Monitoring Board stopped the trial because there was no benefit on survival compared to placebo.
-
Takeaway: LINE-1 inhibition may not be potent enough to stop aggressive diseases like ALS, or the cocktail used (Triumeq) introduced confounding toxicities.
4. Safety & “Biohacker” Considerations
While Lamivudine is considered “safe” compared to older HIV drugs (like AZT), it is not risk-free for healthy longevity use.
-
Mitochondrial Toxicity: All NRTIs inhibit DNA Polymerase Gamma, the enzyme responsible for replicating mitochondrial DNA. While Lamivudine is a weak inhibitor, long-term use can theoretically lead to mitochondrial dysfunction (the very thing you want to avoid in aging).
-
Bone Health: HIV cohorts on Lamivudine (specifically the ABC/3TC combination) generally show better bone density than those on Tenofovir, but osteopenia remains a risk in long-term NRTI therapy.
-
Dosing: The human trial used 300 mg/day .11 Longevity protocols often speculate on lower “pulsed” dosing to mitigate mitochondrial risk, but this is untested.
Summary of Evidence Hierarchy
-
Strongest Support: Mechanisms of aging (Inflammation/SIRT6) in mice.
-
Moderate Support: Neuroprotection biomarkers in early Alzheimer’s (Humans).
-
Weak/Negative Support: Survival extension in aggressive human disease (ALS).
Verdict: Lamivudine is a potent tool for reducing sterile inflammation (Inflammaging). It is currently best viewed as an experimental intervention for specific neuro-inflammatory conditions (like early Alzheimer’s) rather than a general “life extension” pill for healthy adults, pending data on long-term mitochondrial safety.