For decades, the standard of care for preventing heart attacks has relied on a “risk factor” casino—using calculators (like Framingham or ASCVD scores) to guess your probability of an event based on age, cholesterol, and smoking status. This 2019 State-of-the-Art Review by Ahmadi, Narula, and colleagues declares that model obsolete. Their core thesis disrupts the “Vulnerable Plaque” dogma, which assumed heart attacks occur when mild, non-obstructive lesions suddenly rupture.
Instead, the authors provide compelling evidence for the “Plaque Progression” model. Analyzing data from serial angiograms and intravascular ultrasound (IVUS) taken months before heart attacks, they found that “mild” plaques do not just randomly rupture; they undergo a phase of rapid, voluminous progression immediately preceding the event. The plaque grows, the necrotic core expands, and the fibrous cap thins—a process that is visible on imaging months before the attack.
The “Big Idea” here is a shift from treating probability to treating pathology. The authors argue that if we use modern imaging (Coronary CTA) to detect subclinical atherosclerosis early, we can use intensive lipid-lowering therapy to mechanically halt plaque progression. The data shows that when LDL is driven below 70 mg/dL ( <1.8mMol/L), and ideally lower, plaque growth stops, and the risk of rupture drops near zero. This suggests that the current distinction between “Primary Prevention” (before a heart attack) and “Secondary Prevention” (after a heart attack) is an artificial administrative boundary. The only biological distinction that matters is: Do you have plaque, and is it growing?
Source:
- Open Access Paper: From Subclinical Atherosclerosis to Plaque Progression and Acute Coronary Events: JACC State-of-the-Art Review
- Institution/Journal: The Icahn School of Medicine at Mount Sinai (USA), St. Paul’s Hospital (Canada), and University of Edinburgh (Scotland); published in the Journal of the American College of Cardiology (JACC).
- Impact Evaluation: The impact score of this journal is 22.3. Therefore, this is an Elite impact journal, representing the highest tier of cardiovascular consensus.
Part 2: The Biohacker Analysis
Review Scope & Evidence Base
- Type: State-of-the-Art Clinical Review (Meta-synthesis of serial angiography, IVUS, and CTA studies).
- Subjects: Humans. The review aggregates data from major trials like PROSPECT, PARADIGM, and GLAGOV.
- Key Insight: In the PROSPECT study, non-culprit lesions that caused future events were mild at baseline (32% stenosis) but rapidly doubled in size (to ~65%) before rupturing. This “rapid progression phase” is the window of opportunity for intervention.
Mechanistic Deep Dive (Longevity Lens)
- The Necrotic Core Driver: The study identifies the expansion of the “necrotic core” (dead cellular debris inside the artery wall) as the primary fuel for progression. This is a failure of autophagy and efferocytosis (clearing dead cells).
- Lipid-Driven Inflammation: The rapid growth is fueled by LDL cholesterol infiltration. Lowering LDL removes the fuel source.
- Plaque Stabilization (Calcification): Crucially, the paper notes that successful statin therapy often increasescoronary calcium scores. This is not progression of disease, but a transformation of “soft,” rupture-prone fatty plaque into “hard,” stable calcified scar tissue. This distinction is vital for biohackers tracking their own Calcium Scores.
Novelty (The “New” Knowledge)
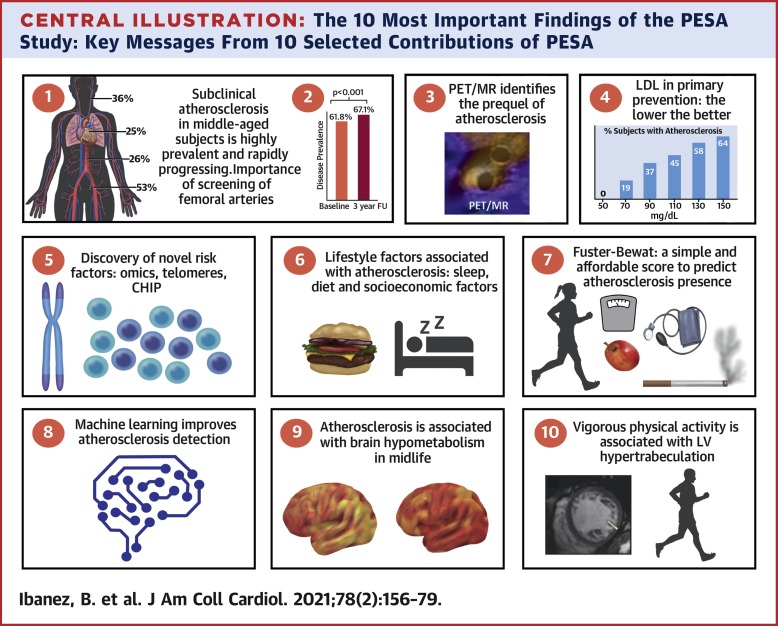
The paper dismantles the idea that “low risk” patients (by standard calculators) are safe. It cites the PESA study, showing 50% of “low risk” individuals have subclinical atherosclerosis. The novelty is the proposal of a new clinical standard: “Preponing” Secondary Prevention. Treat anyone with visible plaque as if they have already had a heart attack.
Critical Limitations
- No Randomized Control Trial (RCT) for Strategy: While the drug data is from RCTs, the specific strategy of “Screen everyone with CTA and treat based on plaque regardless of risk score” was a proposal, not a tested trial outcome in this paper (though SCOT-HEART later supported this).
- Radiation Exposure: The reliance on serial Coronary CTA implies repeated radiation exposure, which may be a concern for younger longevity enthusiasts, though modern scanners have very low doses.
- Cost-Benefit Gap: Mass screening with CTA is expensive and not currently reimbursed for asymptomatic people in many systems.
Part 3: Actionable Intelligence
The Translational Protocol: “Halt the Progression”
- Target: The biological goal is to freeze plaque volume and induce “phenotypic stabilization” (thickening the fibrous cap).
- The “Stop” Signal: The paper cites data showing plaque progression halts at LDL levels between 70–80 mg/dL. Plaque regression (shrinkage) typically requires LDL <50 mg/dL.
1. Pharmacological Intervention (Standard of Care +)
-
Primary Agent: Rosuvastatin (5–40 mg) or Atorvastatin (20–80 mg).
- Mechanism: HMG-CoA reductase inhibition; stabilizes plaque, lowers inflammation (hsCRP).
- Safety: Extensive long-term safety data.
-
Adjunct Agent: Ezetimibe (10 mg).
- Use Case: Added if Statin alone does not reach LDL < 70 mg/dL. Inhibits cholesterol absorption.
-
Elite Intervention: PCSK9 Inhibitors (Evolocumab/Alirocumab).
- Use Case: For aggressive regression or statin intolerance. Can drive LDL to <20 mg/dL.
- Cost: High (~$400–$600/month if not covered), but profoundly effective.
- Biohacker Note: The GLAGOV trial showed distinct plaque regression with Evolocumab added to statins.