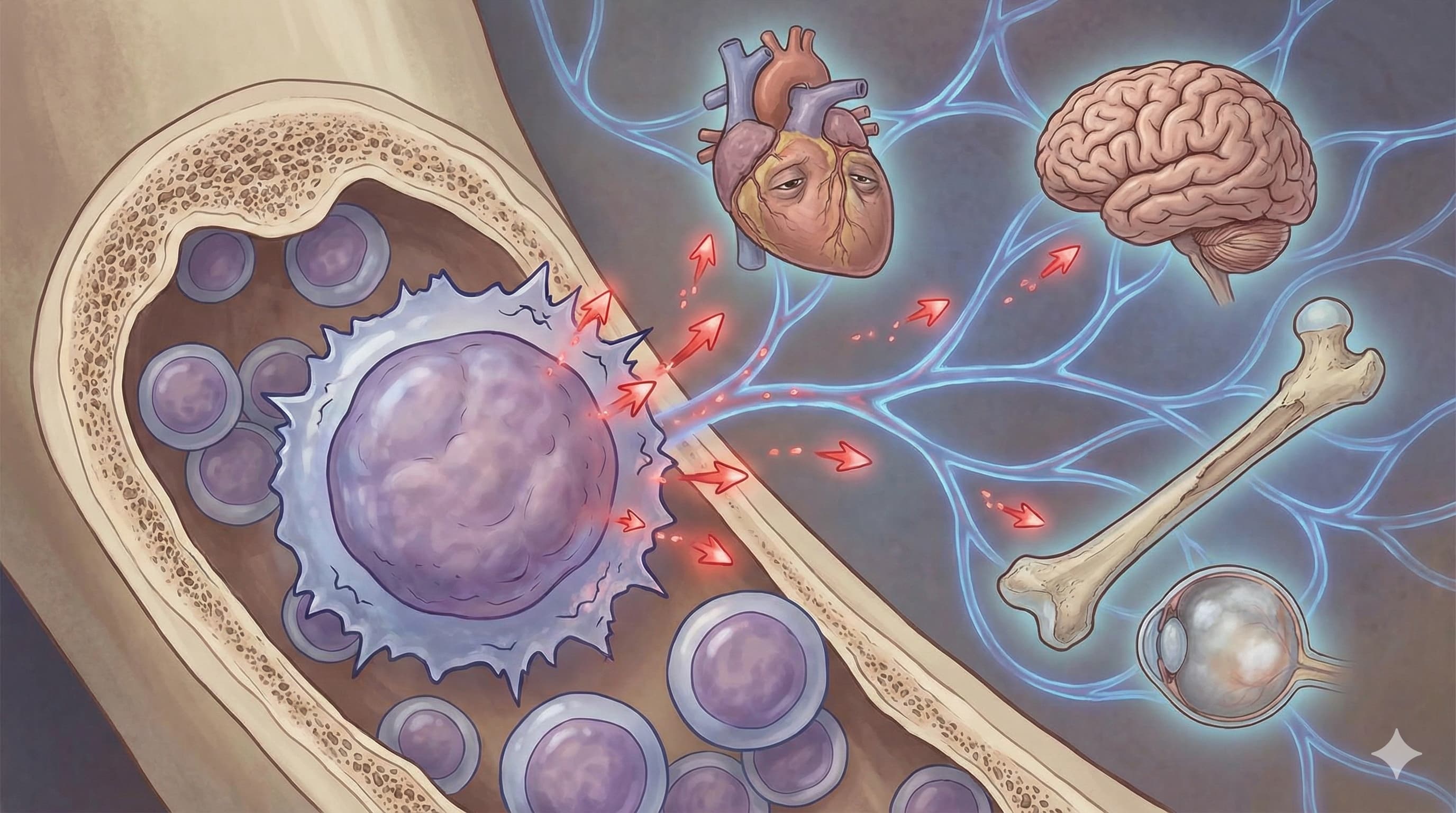
In a compelling new perspective published in FEBS Letters, researchers from the University of Helsinki and Karolinska Institutet, led by Dr. Jette Lengefeld, propose a paradigm shift in longevity science: the “elixir of life” lies in the physical size and clonal quality of Hematopoietic Stem Cells (HSCs). Moving beyond generic “blood rejuvenation” concepts (like parabiosis), this paper crystallizes HSCs as the distinct “Zero Point” of aging. The core argument rests on the “Cellular Enlargement” theory—the observation that HSCs paradoxically grow larger as they age, a hypertrophy that dilutes cytoplasmic factors, disrupts organelle transport, and drives functional failure.
The authors argue that the decline of the immune and blood system is not just a symptom but a cause of systemic organ failure (heart, brain, muscle). By targeting the specific mechanisms of HSC aging—specifically preventing cellular enlargement (via mTOR suppression) and correcting polarity defects (via Cdc42 inhibition)—we can potentially reset the body’s entire regenerative clock. This review synthesizes evidence that rejuvenating HSCs alone can extend lifespan and healthspan, positioning the bone marrow niche as the highest-ROI target for longevity interventions.
Context:
- Institution: University of Helsinki (Finland) / Karolinska Institutet (Sweden)
- Journal: FEBS Letters
-
Impact Evaluation: The impact score of this journal is ~3.0 (2024 JIF), evaluated against a typical high-end range of 0–60+ for top general science (e.g., Nature, Cell), therefore this is a Medium impact journal. It is a respectable, specialized venue for molecular biosciences.
Open Access Paper: Hematopoietic (stem) cells—The elixir of life?
Related: Rapamycin Prevents Blood Stem Cell Aging, New MIT Study
Part 2: The Biohacker Analysis
Study Design Specifications:
- Type: Review & Perspective Article (Synthesizing previous in vivo mouse data and human clinical observations).
- Subjects: Discusses data primarily from Mus musculus (C57BL/6 strains) and human clinical cohorts (bone marrow transplant data).
- Lifespan Data: Cites specific intervention data (e.g., Cdc42 inhibition via CASIN) demonstrating ~10-15% lifespan extension in aged mice, and “young-like” immune restoration. (Note: This specific paper is a review of these findings, not the primary trial itself).
Mechanistic Deep Dive: The paper pivots from “molecular damage” to “physical biophysics” as a driver of aging:
- HSC Hypertrophy (The Lengefeld Mechanism): Old HSCs are physically larger than young ones. This enlargement is driven by a decoupling of cell growth (mass accumulation) from cell division. Dysregulated mTORsignaling drives this mass increase.
- Consequences of Size: Enlarged cells suffer from “Cytoplasmic Dilution”—transcription factors and metabolites become too dilute to function effectively.
- Cdc42 & Polarity: Elevated activity of the RhoGTPase Cdc42 in aged HSCs destroys their polarity. Instead of dividing asymmetrically (keeping one stem cell, making one blood cell), they divide symmetrically, leading to stem cell exhaustion.
- Organ-Specific Priority: The review establishes the Bone Marrow as the priority organ. Aging here exports inflammation (myeloid bias) to the heart, brain, and gut.
Novelty: The “Big Idea” is the shift from genetic mutations to cytoskeletal/biophysical changes (Size and Polarity) as the root cause of HSC aging. It suggests that keeping stem cells physically small (via Rapamycin or similar) is a more potent preservation strategy than previously realized.
Critical Limitations:
- Translational Uncertainty: The primary specific rejuvenator discussed (CASIN) is a research chemical with no human safety data.
- Methodological Gap: Measuring HSC size in living humans is invasive (requires bone marrow biopsy), making it a difficult biomarker for biohackers to track.
- Effect Size: While “rejuvenation” is claimed, the absolute lifespan extension in cited mouse models (via CASIN) is modest compared to caloric restriction, suggesting HSCs are a necessary but perhaps not sufficient single target for radical life extension.
Part 3: Actionable Intelligence
The Translational Protocol
Intervention A: Rapamycin (The Size Regulator) Rationale: To inhibit mTORC1, preventing the “Cellular Enlargement” of HSCs.
-
Human Equivalent Dose (HED):
- Animal Data: Murine studies for HSC size control often use intermittent high-dose or chronic low-dose Rapamycin. (e.g., 2 mg/kg in mice).
- Calculation: 2 mg/kg (Mouse) ÷ 12.3 (Km Factor) = ~0.16 mg/kg Human.
- For a 75kg Human: ~12 mg (Weekly pulse) or ~1 mg (Daily).
- Note: The “Standard” anti-aging protocol (5-6 mg weekly) aligns well with HSC size suppression without causing immune suppression.
- Pharmacokinetics: Bioavailability ~14%. Half-life ~62 hours.
-
Biomarker Verification:
- Target Engagement: Reduced pS6 (phosphorylated S6 ribosomal protein) in PBMCs (Peripheral Blood Mononuclear Cells).
- Downstream: Lower RDW (Red Cell Distribution Width) – high RDW is a proxy for anisocytosis and poor HSC quality.