This research paper is from 2021, but I thought it would be of interest to people here:
Broccoli’s Master Molecule Reverses Skin Aging at the Genetic Level
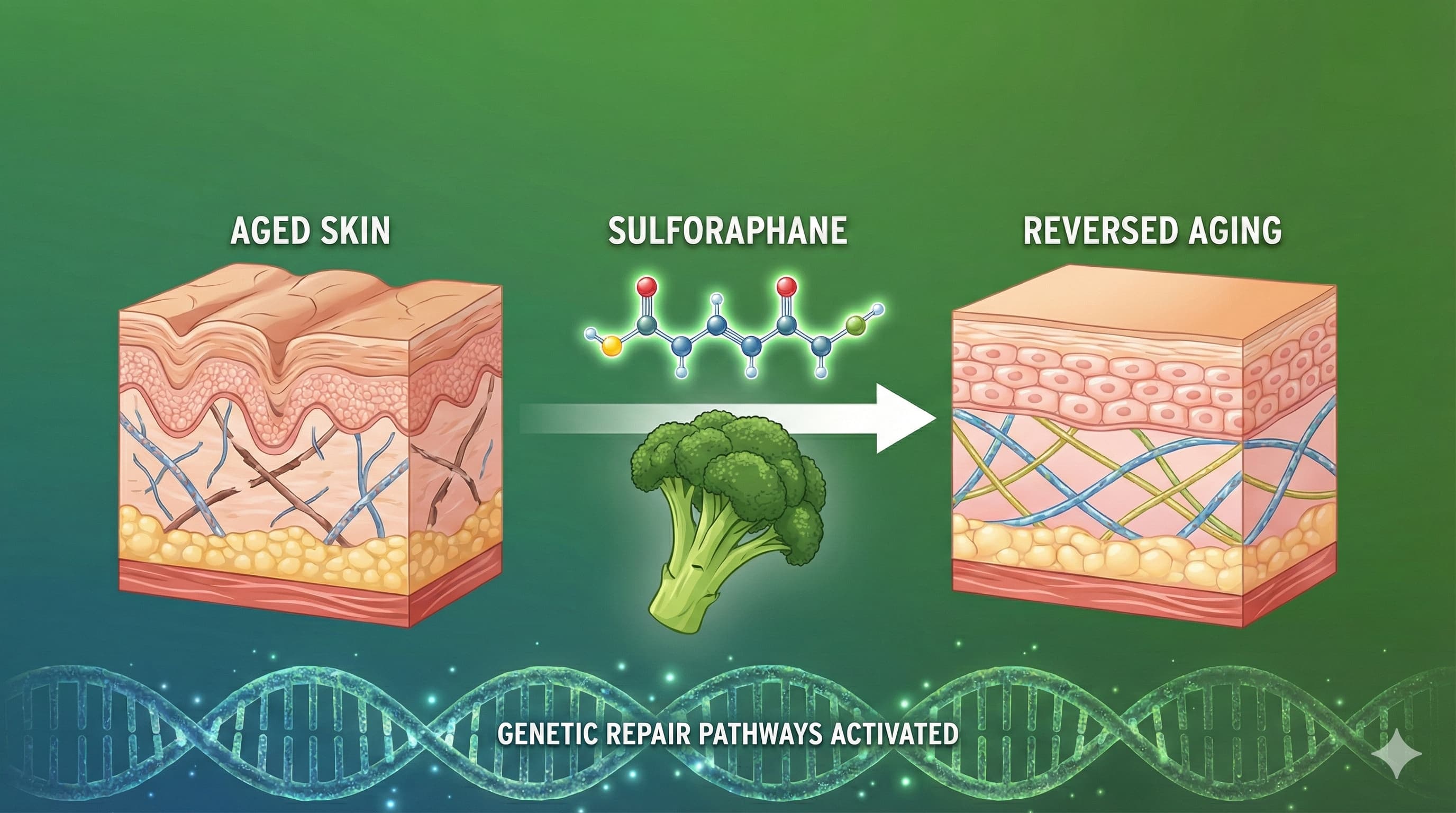
In a compelling demonstration of “food as medicine,” researchers have reversed biological markers of skin aging in mice using a high-dose dietary regimen of Sulforaphane (SFN), the potent bioactive compound found in broccoli. While the cosmetic industry obsesses over topical creams, this study reveals that systemic, internal activation of the Keap1-Nrf2 pathway—the body’s master antioxidant switch—can physically restructure the dermal layer from the inside out.
The study compared young (2-month) and old (21-month) mice, finding that the elderly cohort suffered from collapsed collagen networks and high oxidative stress. However, when these “geriatric” mice were fed a diet fortified with SFN for three months, their skin’s genetic profile began to mimic that of youth. The treatment successfully restored collagen deposition and upregulated critical defense genes (like NQO1 and HO1) that usually go dormant with age. Crucially, this wasn’t just about reducing inflammation; it was a structural remodeling of the extracellular matrix, suggesting that longevity compounds could make our largest organ—the skin—functionally younger, not just smoother.
Context:
- Institution: University of Coimbra (Portugal) & Texas Tech University Health Sciences Center (USA).
- Journal: Journal of Nutritional Biochemistry.
- Impact Evaluation: The impact score of this journal is ~4.9 (2024 JIF), evaluated against a typical high-end range of 0–60+ for top general science, therefore this is a Medium impact journal. (While not Nature or Science, it is a respected, specialized tier-1 journal in nutritional science).
Part 2: The Biohacker Analysis
Study Design Specifications
- Type: In vivo (Animal Study).
-
Subjects: Male C57BL/6 mice.
- Groups: Young (2 months) vs. Old (21 months).
- Treatment: Sulforaphane (SFN) incorporated into the diet at 442.5 mg/kg of diet for 3 months.
- Lifespan Data: Not Measured. The study was terminated after 3 months to harvest tissue. No longevity survival curves were generated.
Mechanistic Deep Dive
The study targets the Nrf2 (Nuclear factor erythroid 2-related factor 2) pathway, often called the “guardian of longevity.”
- The Problem: In aging skin, Nrf2 gets trapped by a protein called Keap1 and degraded, preventing it from entering the nucleus to activate defense genes. This leads to ROS (Reactive Oxygen Species) accumulation and collagen degradation via MMP9.
- The Fix: SFN modifies Keap1 cysteine residues, liberating Nrf2.
-
Key Findings:
- Gene Expression: Old treated mice showed a massive resurgence in antioxidant mRNA (Nrf2, NQO1, HO1), restoring levels comparable to young mice.
- Collagen: Masson’s trichrome staining revealed that SFN significantly improved collagen structural integrity in old mice.
- Mitochondrial/ROS: Protein levels of ROS and MMP9 (an enzyme that chews up collagen) were significantly suppressed.
Novelty
Most skin aging studies focus on topical application (creams/serums). This paper validates systemic, dietary intervention for dermal remodeling. It proves that skin aging is a metabolic issue that can be addressed centrally via the gut-skin axis, specifically utilizing the Nrf2 pathway to reverse established structural damage, not just prevent it.
Critical Limitations
- Supra-Physiological Dosing: The dose used (442.5 mg/kg diet) translates to a massive human equivalent dose (see Part 3). This is likely unachievable via eating broccoli alone.
- No Functional Lifespan Data: We do not know if these mice lived longer, only that their skin looked better.
- Dermal Thickness Gap: While collagen quality improved, the actual thickness of the dermis in old mice did not fully recover to young levels, suggesting a “ceiling” to the regenerative effect.
- Gender Bias: Only male mice were used. Estrogen plays a massive role in skin aging (collagen production); excluding females is a major translational blind spot.
Part 3: Actionable Intelligence
The Translational Protocol (Rigorous Extrapolation)
WARNING: The dose extrapolated below is considered HIGH and potentially toxic for humans if taken as a pure isolate. Proceed with extreme caution.
1. Human Equivalent Dose (HED) To translate the mouse dose to humans, we use Body Surface Area (BSA) normalization factors (Reference: FDA Guidance for Industry).
- Mouse Input: 442.5 mg SFN per kg of diet.
-
Daily Intake Estimate: A standard mouse consumes approximately 150g of food per kg of body weight (BW) per day.
- Math: 442.5 (mg/kg diet) x 0.15 (kg food/kg BW) = 66.4 mg/kg BW/day (Mouse Dose).
-
HED Calculation:
- Formula: Mouse Dose / 12.3 (Conversion Factor).
- Math: 66.4 / 12.3 = 5.4 mg/kg BW.
-
For a 75kg Human:
- Math: 5.4 x 75 = ~405 mg/day.
- Context: Most clinical trials for autism or prostate cancer use 15–60 mg/day. A 405 mg dose is roughly 6x to 10x higher than standard therapeutic doses.
2. Pharmacokinetics (PK/PD)
- Bioavailability: Sulforaphane has high absolute bioavailability (~80%) but a very short half-life.
- Half-Life: Approximately 1.8 to 2.5 hours in humans. (Source: PubMed - Pharmacokinetics of SFN).
- Implication: To mimic the continuous dietary intake of the mice, a human would need Pulsed Dosing (e.g., 100mg every 6 hours). Single daily dosing is insufficient for sustained Nrf2 activation.
3. Safety & Toxicity Check
- Toxicity Signals: In rat toxicity studies, sedation, hypothermia, and motor impairment appear at doses >150 mg/kg. The calculated HED (5.4 mg/kg) is below the lethal threshold but enters the range of potential hepatotoxicity and gastrointestinal distress. (Source: ToxNet / PubChem Data).
- Interactions: SFN is a potent inhibitor of CYP3A4. It may alter the metabolism of statins, benzodiazepines, and immunosuppressants.
4. Biomarker Verification Panel
- Efficacy: Do not rely on “feeling better.” Test GGT (Gamma-Glutamyl Transferase)—low levels correlate with good glutathione status. Advanced users can test Urinary Mercapturic Acids (SFN-NAC) to verify absorption.
- Safety Monitoring: Liver Panel (ALT/AST) is mandatory at these doses. Watch for thyroid suppression (goitrogenic effect of high-dose crucifers).
5. Feasibility & ROI
- Sourcing: Pure “Stabilized Sulforaphane” is rare and expensive. Most supplements are “Glucoraphanin + Myrosinase” (precursor blends).
-
Cost Estimate:
- To hit ~400mg SFN using high-end supplements (e.g., Broc Shot @ 12mg/dose) would require ~33 doses/day.
- Cost: >$2,000/month.
- Verdict: Economically Unfeasible for this specific study protocol. Users must rely on lower, optimized doses (e.g., 30-50mg) combined with synergistic Nrf2 activators (Curcumin, Resveratrol) to achieve similar effects.
Source Research Paper (Open Access): Dietary supplementation with sulforaphane ameliorates skin aging through activation of the Keap1-Nrf2 pathway