“Imagine that every time you were doing IND-enabling studies, you could also run a lifespan study in mice,” said the paper’s lead author, Insilico CEO Alex Zhavaronkov. “Every pharma program hits the 12-month IND-enabling window. Why not use that same year to run a parallel mouse lifespan study?”
The only problem is cost.
However, I love the idea.
Wouldn’t be that expensive compared to what companies spend. It would also be very good data.
Having for decades observed how frequently studies on mice fail to generalize to humans, I’m on the other side of this issue. ITP, for example, is a worthwhile but overinterpreted initiative.
Ok so it’s basically happening. Even better. Slight alterations to these types of tests can make them suitable for lifespan analysis.
Now if someone wanted they could (1) download all of the pharmacology documents for all the drugs in the Drugs@FDA database (2) extract the data for every 104 week (2-year) mouse carcinogenicity (lifespan?) study from the pdf’s, from charts or tables.
We’d have a list of most drugs that deemed necessary to do such a study (maybe taken regularly) how they affect mouse lifespan over 2 years and sort the drugs with the largest effect.
Can we assume that all drugs, in regards to life extension, behave the same in mice as in humans?
I just looked this up. Looks like it’s an abortion drug for women? I’m dying to know the mechanism.
Could also be a massively powerful and high impact paper. @Krister_Kauppi @Agetron @RapAdmin - any of you want to run the idea by Matt K and see if he’d like to do it?
Pretty sure Matt keeps up on this site. He chimes in time to time…
You could PM him… here.
It could be a good project for the community here to work on as well. If we can figure out how to find this data in a consistent manner we can begin compiling it.
@Krister_Kauppi runs a lifespan database (Rapamycin Longevity Lab - It's time to speed things up!) and he would possibly be willing to put this data in if it is valid.
@Neo & @AustraliaLongevity Great idea, we could definitely add that data to the LID database and carcinogenicity tag the compounds. Just reach out if someone is interested in gathering this data then I can show how I usually do when collecting data from different studies. I’m currently working on the Omipalisib (GSK2126458) paper and gathering lifespan data from different places to this paper. All that data will be added to the LID database in July or August.
This is my take also. ITP is amazing, but I wouldn’t take rapamycin (for example) if there weren’t longevity studies in other model organisms… AND even then, taking rapamycin in any dose really is a leap of faith.
Requiring longevity studies in mice might be good for screening molecules for future research, but won’t provide any direct benefit for end-users.
It would provide another piece of evidence towards a longevity treatment.
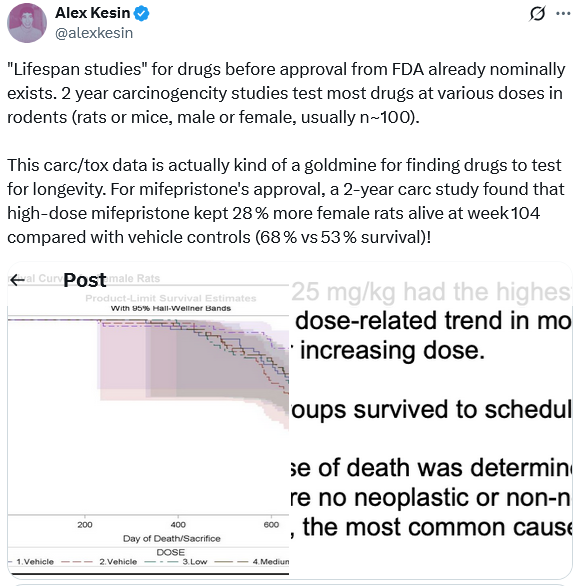
This guy provides a link to a document on the FDA website on mifepristone.
What is a way we can access this document on any drug/molecule on the FDA website?
Once we access this document, what is the most effective way we can get lifespan relevant data on that drug/molecule?
Let’s create a standard operating procedure of extracting this data.
Thanks for looping me in. I don’t typically see what gets posted here - it’s an interesting discussion. Have a few thoughts. (1) I agree with Alex’s initial concept and this could easily be done in mice at relativley low cost (compared to the cost of moving an unworthy drug forward) in less than a year by starting in 24 month old mice. But people would need to be disciplined and only follow up with things that have big effects, assuming the goal is a geroprotector, not an incremental disease drug. (2) Lifespan studies are not carcinogenicity studies. Perhaps that wasn’t what the comment intended, but this is what it sounded like. This requires more time to get into than I’m willing to spend here, but I’ve covered it on the podcast. Curing cancer has a small effect on lifespan in mice - or shortens it because of massive cell death due to hyperactive clearance - and does not impact other age-related functional declines/diseases. The interventions that robustly increase lifespan in mice tend to delay but not cure cancer and also delay most/all declines/diseases of aging. (3) The number of drugs with robust positive lifespan effects is very small and the reproducibility problem is large (e.g. NR, AKG, fisetin, metformin, etc. etc.). I think we already know all the drugs that have solid lifespan data in mice, so I’m not sure what new discovery would be made going that direction. Perhaps I’m missing something about the overarching goal, however. (4) The idea of starting from carcinogenicity studies to find candidates for longevity studies is intriguing. We could likely get an estimate of how effective this would be from drugs where we already have good data for both and see how strong the correlation is. I don’t know the answer. I don’t have the bandwidth to do it myself, but happy to help provide thoughts if someone wants to take it on.
Thanks Matt!
You provide an excellent perspective on getting solid data. This clarifies the intent and provides a great jumping off point for research.
By the way, I really enjoyed your Optispan interview with Dr. Fahy. You asked solid questions… dug deep and really helped him explain the important steps and results.
At 67.5 years on rapamycin since 2020. Now going on 5 years (with amazing test and physiological results)… the podcast with Fahy inspired me to visit with my personal physician and do his protocol. I started in mid-January 2025. After 5 months daily use… have to say really feeling great and seeing excellent body changes… skin particularly and muscles. Looks like you might be trying it. You should.
Your first podcasts on the Drive with Attia really pushed me to go all in on rapamycin. For me, it was a successful health span and longevity decision.
You also, in my early months, suggested joining rapamycin.news for sharing and getting current information on rapamycin. Another helpful bit of information.
Keep up your important research work.
We all have benefitted. Definitely our Rapamycin Rock Star.
![]()
No
Mice are because they are about as far up the lifespan curve as we can reasonably experiment with. Mammals with longer lifespans are more expensive in almost every way to experiment with. Mice are a good screening device to move on to longer-lived mammals.
I find it disheartening that, to the best of my knowledge, nothing has doubled the median or maximum lifespan in mice.
So, at this point, using rapamycin, etc., may be doing little more than extending my healthspan.
At this point, that’s my goal. Extended lifespan would be a bonus.