So Steve_C what is your exact Protocol?
And can you provide link to the Quercetin you use?
I use “regular” Quercetin but in my little formula there are a number of bio-availability enhancers. So it’s a bit more complicated than just Quercetin.
There are 9 compounds in our formula, all to enhance the performance of the 2 key compounds, Quercetin and Fisetin.
One could always purchase the brand used in the successful trials, Thorne.
Also I’d be happy to offer anyone on this forum a discount off our product of 80%
I modelled it on the protocol used in the 2 clinical trials but taking into consideration we are not using Dasatinib.
Depending on age and how long a person has been clearing their senescent cell, it could be 1 cycle per year or up to 4 cycles per year. We do 4 per year and have done so since 2019.
1 cycle = is 4 weeks of 3 days on, 4 days off. It’s a very high volume of compounds with a relatively short half-life. This leads to 3 times per day consuming the formula. By doing this it helps to keep the blood plasma levels of the compounds “high” for longer over the 3 day period.
So the formula and protocol are a bit more and a bit longer ![]()
We do use both Quercetin and Fisetin in our product. It’s a combination type of concept. We know that Q is not the best senolytic, we know that Fisetin is “better” but we also know that those polyphenols are not very bio-available.
My dev process has always been to look at the weaknesses/deficiencies and find ways to overcome those. And at the same time, look for synergistic opportunities.
My background is more hardware oriented but the same principles applied when I was developing medical devices.
can you provide a link?
How did you decide on 6 grams?
I decided on 6 g of taurine daily after watching the video guide on Taurine (see link below). The author later updated his recommendation to be a number of grams equal to your age/10. So, I’ve reduced my intake to 4 g daily.
Meanwhile, Unity is moving ahead with their senolytic therapy in a new clinical trial. If you know anyone with this eye condition - send them the information for to enroll:
Assess the Efficacy and Safety of Repeat Intravitreal Injections of Foselutoclax (UBX1325) in Patients With DME (ASPIRE)
https://clinicaltrials.gov/study/NCT06011798?cond=DME&intr=UBX1325&rank=2
Mice Cleared of Senescent Cells Shown to Die Healthier—Not Just Live Longer
By treating a particular brand of senescent cells, it is now possible to live healthier for longer—at least in mice. If the findings can be translated to humans, that could mean an extra decade of healthiness. Plenty of medicines are already available to help people live longer, but not necessarily with vitality, according to gerontologist Ming Xu, Ph.D., assistant professor in the University of Connecticut’s Center on Aging and the department of genetics and genome sciences.
For the most part, anti-aging researchers have been measuring physical functioning only at certain time point and “not comprehensively to assess the dynamic of the healthspan over the post-treatment lifespan,” says Xu. That deficiency was uniquely addressed with a time-consuming study, published in Cell Metabolism (DOI: 10.1016/j.cmet.2024.07.006), where the health of individual mice was assessed monthly from the time they were 20 months old (equivalent to 60-year-old humans) until death.
Full article:
I guess the issue for me is what is the best senolytic therapy, and is it worth doing a senolytic if you are already taking senomorphics such as Rapamycin and/or Taurine.
A key thing to me is how easily to measure the burden of senescent cells. I think the baseline CRP gives a good idea of this remembering that CRP is raised by infection so you cannot rely on a single measurement, but need to take a number of data points and look for the minimum.
SapereX appears to be the best test to date (but not available to the consumer). But according to the founder, Natalia Mitin (@nym ) ,
"as someone whose company measures senescence in humans with an analytically- validated assay that took 6 years to develop and has now been performed on over 7000 human samples of people doing all sorts of interventions, there are two things i can say:
senescence in blood is not physiologically good except for low levels needed for the tumor suppression
there is no evidence that supplements sold as senolytics decrease senescent cells in blood in humans. that diabetic kidney study from Kirkland collected blood but reported changes in fat and skin. my conclusion is that data in blood was negative. if senescence in blood is unchanged, improving it in a fat pad will not likely give you any functional or certainly longevity benefits
i know we all want senolytics but currently, your best bet is either indirect senolytics (improving immune system function for natural senolytic effect) or preventing senescence by adopting a healthy lifestyle and taking care of your mitochondria (e.g. rapamycin, but senescence prevention is impossible to prove in a human trial)"
https://www.rapamycin.news/t/senescent-cells-heads-i-win-tails-you-lose/14595/13?u=ng0rge
Nice reminder. I have taken her advice to heart. I make no effort to directly target senescent cells. I focus on lifestyle interventions such as exercise and, now, periodic FMD. Rapa is my Hail Mary.
I’ll wait for good Senolytics to show up or be proven.
glad to hear! we are collecting data as fast as we can on all sorts of longevity treatments. will be presenting these thoughts at ARDD next week. still strongly believe that addressing immune imbalances, if present, with e.g. rapa is the safest way to go about targeting senescence at this time
Amazing. I never heard half of this paper put out by Lamming’s lab. I copied some snippets below:
https://www.sciencedirect.com/science/article/pii/S1550413124003279
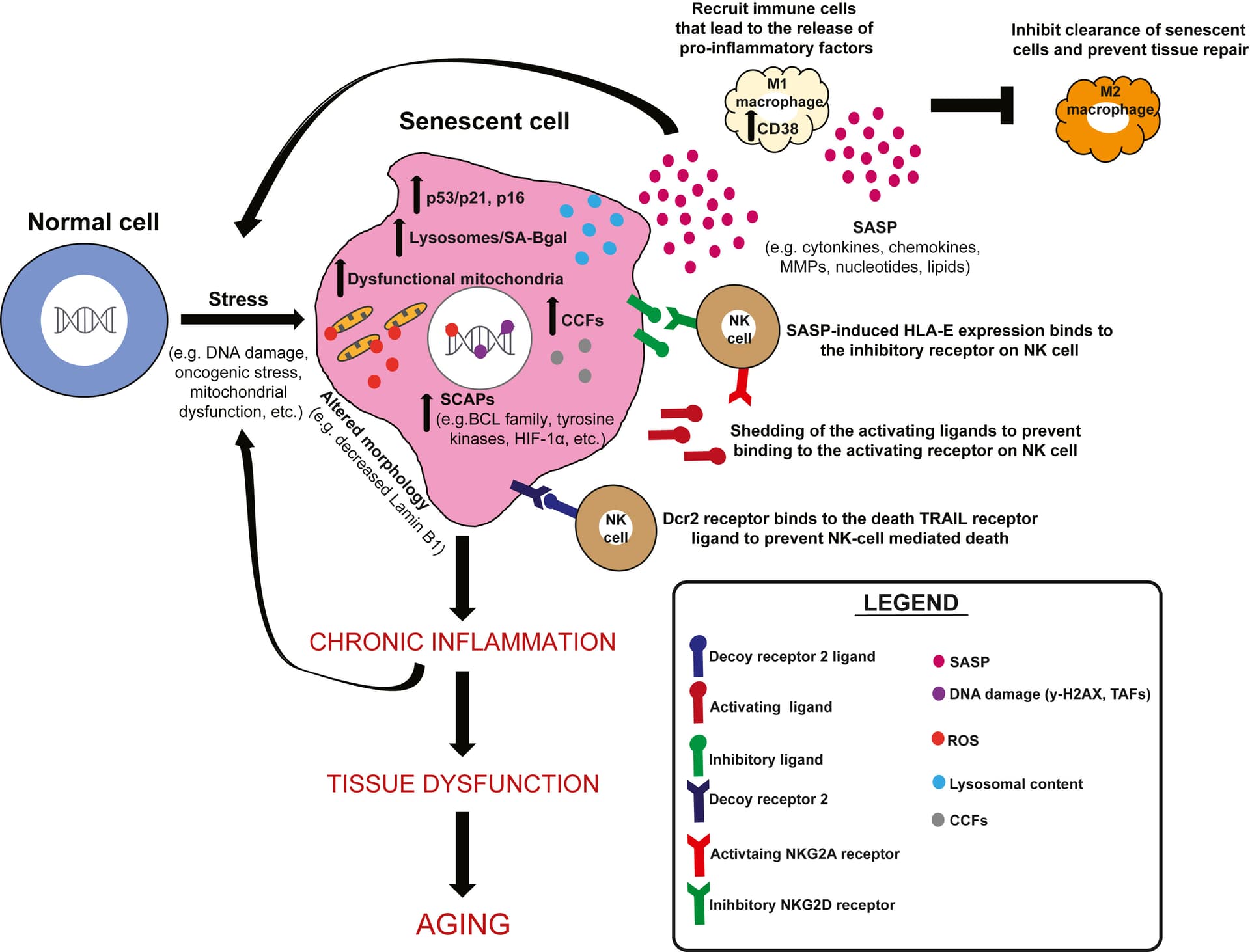
This review will focus on nutritional modulation of senescence, as exciting new evidence suggests aging and age-related diseases driven by senescence can be regulated by diet.
CR for 18 weeks decreases circulating senescence biomarkers in aged individuals. CR protects against senescence in multiple tissues of mice, rats, and humans.
Dietary protein and specific amino acids promote senescence. Methionine, arginine, BCAA. Taurine, proline reduce senescence. While there have been only a few studies examining how protein effects senescence, a recent study found that expression of p16 and p21 in the liver is associated with protein consumption,153 and PR reduces IL1α and TNFα in the livers of old male mice.154
Simple sugars, such as glucose, fructose, and galactose, promote senescence in culture and mice. Complex starches may be able to protect against senescence.
Increased dietary fat promotes senescence in multiple tissues of multiple model organisms to humans.
Micronutrients also play a role in organismal aging and senescence, particularly with suppression of SASP.245 Vitamin E has been shown to delay senescence in human umbilical vein endothelial cell (HUVEC) and fibroblast culture.246 Vitamin D decreases NF-κB activity in murine macrophage cells, suggesting it may reduce SASP.247
MR decreases senescence,156,157,169.
Outside of vitamins, polyphenols derived from cocoa have been shown to inhibit senescence via the modulation of sirtuins in auditory cell lines.249 Procyanidin C1, which is a polyphenolic component of grape seed extract (GSE), reduces SnC burden and increases the healthspan and lifespan of mice.250,251 In terms of minerals, chronic exposure to high-dose zinc can also accelerate senescence in human endothelial cells.252 Magnesium deficiency accelerates senescence in cultured cells,253
Protein and glucose restrictions have also been shown to activate AMPK.54,313 By contrast, high-fat diets inhibit AMPK.220,314Downregulation of AMPK activity induces premature senescence, while AMPK activation prevents premature senescence in MEFs, NIH3T3 cells, dental follicle cells, tendon stem/progenitor cell, human keratinocytes via maintenance of metabolic homeostasis, and murine skin.315,316,317,318,319,320 Conversely, AMPK induces senescence in endothelial cells, colorectal carcinoma cells, and fibroblasts.268,321,322 Persistent activation of AMPK accelerates senescence in a p53-dependent manner.323
IGF-1 itself also induces senescence in human fibroblasts, MEFs, 2BS cells, and mouse hepatic stellate cells in a p53-dependent manner327,328,329,330,331 but may decrease senescence in endothelial progenitor cells from older humans.332
The dietary level of specific macronutrients regulates mTORC1 activity, with restriction of protein or BCAAs leading to lower mTORC1 activity.148,152,343,344 Thus, nutrients can impact mTORC1-mediated senescence. Acquisition of senescence leads to constitutive upregulation of mTORC1 in human fibroblasts, making it resistant to both serum and amino acid starvation.345 High-protein diets, which promote mTORC1 activity in vivo in mice, increase levels of senescence in the liver of C57BL/6 male mice.153 In human HepG2 cells, BCAA supplementation enhances premature senescence via mTORC1.174 In addition, 16-month-old male C57BL/6J mice given dietary BCAA supplementation for 12 months had increased pro-inflammatory gene expression in visceral WAT,158 some of which overlap with common SASP markers. Consistent with this, BCAA accumulation within the cell promotes mTORC1-mediated SASP production in HEK293T cells.172
Other dietary macronutrients have been shown to activate mTORC1 activity, including carbohydrates. For example, in the context of pancreatic ductal adenocarcinoma, fructose induces mTORC1 activity.346 Galactose also induces mTORC1 activity in MSCs and rodent skin.196,197,198 Similarly, fats activate mTORC1. High-fat diets reduce AMPK phosphorylation, leading to increased mTOR activity
to maximize the therapeutic benefits of current senotherapeutics, diets should be taken into consideration. For example, rapamycin, which inhibits mTORC1 and extends lifespan, has been shown to have greater molecular effects in the context of high-protein diets, while metformin has the greatest molecular impact in the context of low-protein diets.344 This suggests that the drug efficiency may be altered by the diet consumed. While the efficacy of senolytics has been tested in high-fat diets, their interactions with dietary protein or carbohydrates have not been examined.48,207,213,396
it is important to consider the diets in which these individuals are consuming to maximize the benefits. For example, rapamycin, which acts as a senomorphic,402 might have the biggest benefits for those eating a high-protein diet, and negligible benefits for individuals eating a low-protein diet.
Please contact your clinical team at Optispan. Once the rest is commercially available, report will have a lot more interpretation and context, this is a version that is used for clinical research reporting to comply with Institutional Review Board rules.
You only ship to Canada and US correct ?
This is interesting… from Dudley Lamming’s lab:
A nutrigeroscience approach: Dietary macronutrients and cellular senescence
Here, we review what is currently known about dietary macronutrients’ effect on senescence and the SASP, the nutrient-responsive molecular mechanisms that may mediate these effects, and the potential for these findings to inform the development of a nutrigeroscience approach to healthy aging.
Paywalled:
https://www.cell.com/cell-metabolism/abstract/S1550-4131(24)00327-9
Full paper available at this twitter post link: x.com
Yes, that is correct.