Pretty sure you can’t get burned by IR light.
I didn’t get burned, and after about 30 minutes the burning sensation stopped. It felt like a very mild sunburn.
Infrared light can burn. Infrared light is basically heat radiation and too much infrared light can result in the transfer of too much heat which can cause damage.
Should I be experiencing this feeling or should I move the IR source further away? I don’t want to lose the hair left there!
Try moving it away and see how you feel. If it feels too hot, then you’re too close.
The heat from the lamp may be a combination of two sources: 1) heat from the infrared and 2) heat from the light bulbs not being perfectly efficient so some electricity is converted to heat instead of light.
You cannot get a “sunburn” (if the lamp doesn’t emit uv also) but you still can get a physical burn from the heat
I bought a red light/IR hat on Alibaba a year ago. I’ve been using it almost daily for 15 minutes a day. It seems to keep my hair healthy.
I do have some gray hair and I can’t say if the hat has reversed any. I use the hat in the hopes that maybe it can prevent or slow down the pace of graying.
Do you use it every day ? Twice a day? Could you take a picture of the ingredients? Thank you very much
Not all red light is the same, and not all devices are equal. I did a fair bit of research on this a few years ago when I started with redlight therapy, and I settled on one company whose devices and research I trust: https://gembared.com/
I use and recommend Gembared devices. They are reasonably priced for high-quality. Check out their articles for more details.
Just looked at them and to be fair they are the first company I’ve seen giving real light output values:

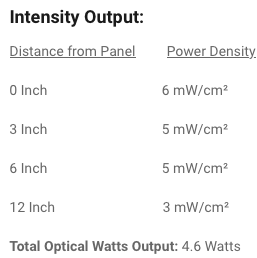
This is totally consistent with the measurements I made on a similar panel and very far from the “Marketing inflated” values of the amazon vendors who claim to have 100mW/cm2 or more.
Here, we demonstrate that murine melanocyte-specific Bmi1 deletion causes premature hair greying and gradual loss of melanocyte lineage cells. Depilation enhances this hair greying defect, accelerating depletion of McSCs in early hair cycles, suggesting that BMI1 acts to protect McSCs against stress. RNA-seq of McSCs, harvested before onset of detectable phenotypic defects, revealed that Bmi1 deletion derepresses p16 Ink4a and p19 Arf, as observed in many other stem cell contexts. Additionally, BMI1 loss downregulated the glutathione S-transferase enzymes, Gsta1 and Gsta2 , which can suppress oxidative stress. Accordingly, treatment with the antioxidant N-acetyl cysteine (NAC) partially rescued melanocyte expansion. Together, our data establish a critical function for BMI1 in McSC maintenance that reflects a partial role for suppression of oxidative stress, and likely transcriptional repression of Cdkn2a.
BMI1 is required for melanocyte stem cell maintenance and hair pigmentation
Fluoxetine promotes human hair follicle pigmentation ex vivo: serotonin reuptake inhibition as a new antigreying strategy?
DEAR EDITOR, The commonly prescribed antidepressant, fluoxetine, has been reported to (rarely) induce epidermal hyperpigmentation after systemic application.1 This selective serotonin reuptake inhibitor (SSRI) can also stimulate melanin production in murine hair follicles (HFs) in vivo under conditions of perceived stress and in cultured human epidermal melanocytes in vitro.2,3 Given the distinct neuroendocrine controls that human HF melanocytes underlie compared with epidermal melanocytes,4and that any pigmentary effects of fluoxetine observed in vivo may have been indirectly mediated, it remains entirely unclear whether fluoxetine impacts directly on human HF pigmentation, specifically in the absence of systemic and neural inputs. In the current pilot study we explored this question after determining the presence of the serotonin transporter and serotonin receptors 1A and 2A at the mRNA and protein levels (data not shown).
Specifically, we asked whether fluoxetine (chosen among other SSRIs for its long half‐life and known pigmentary effect in mice)2,3 can alter the pigmentation of microdissected, organ‐cultured human scalp HFs, using our previously reported HF pigmentation readouts and established protocols.5,6Pigmented, full‐length scalp HFs in anagen VI (obtained from facelift surgery of female donors after written patient consent and ethics approval) were treated for 48 h with 100 nmol L−1 and 1 μmol L−1fluoxetine in serum‐free supplemented Williams’ E medium.5,6 Given the very limited availability of human HFs, the fluoxetine test concentrations were selected based on previously published results.2
Quantitative Masson–Fontana histomorphometry showed that fluoxetine further and significantly increased melanin production in the HF pigmentary unit of already maximally pigmented anagen VI HFs ex vivo (Fig. 1a). This provides the first evidence that fluoxetine can directly stimulate human HF pigmentation in the absence of systemic or neural inputs.
As white HFs occasionally retain a few functionally active melanocytes in their pigmentary unit,7 we next asked whether fluoxetine can stimulate some degree of repigmentation in a very small number of white HFs isolated from five female donors with > 50% canities over a period of ≥ 6 years. These HFs were obtained from 6‐mm punches collected by a contract research organization after receiving written patient consent and ethics approval. Strikingly, fluoxetine induced significant restimulation of intrafollicular melanin production after 6 days of HF organ culture, as measured by quantitative Masson–Fontana histomorphometry (Fig. 1c). This was accompanied by a tendency for upregulation of gp100 immunoreactivity (data not shown), indicating increased premelanosome production and melanocyte activity.5,6 This preliminary evidence in a low number of HFs suggests that at least some canities‐affected HFs can, in principle, be reactivated by fluoxetine to produce melanin
Given that α‐melanocyte‐stimulating hormone (MSH) is a key neuroendocrine stimulator of human HF pigmentation and is synthesized within human anagen scalp HFs,4 and that fluoxetine can modulate the expression and production of α‐MSH in rats and humans,8 we next investigated the effect of fluoxetine on this melanotropic neurohormone. Immunofluorescence microscopy for α‐MSH was performed on anagen HFs and assessed by quantitative immunohistomorphometry in a defined reference area (proximal outer root sheath). This showed that 1 μmol L−1 fluoxetine slightly, but not significantly, stimulated α‐MSH protein expression. While overall this did not reach statistical significance, individual HFs showed a marked increase of α‐MSH immunoreactivity (Fig. 1b). Essentially the same effect was seen in white HFs (Fig. 1d).
Given the low number of HFs we could analyse, the current pilot data are obviously preliminary and require rigorous reproduction in additional HFs from more individuals. However, our preliminary observations strongly suggest that fluoxetine directly promotes human HF pigmentation and may even reverse the depigmentation of at least some ‘white’ female human scalp HFs. Furthermore, the observation that fluoxetine can also upregulate the intrafollicular production of a melanotropic neurohormone (α‐MSH) in some white HFs of women with long‐standing canities suggests that this SSRI may exert longer‐lasting, neuroendocrine changes within white HFs. This would facilitate HF repigmentation by enhancing the capacity of ‘white’ HFs to restore their pigmentation production capacity before the point of no return in HF greying, i.e. when HF melanocyte stem cells have been lost.
The obvious clinical key challenge now is to study whether the observed ex vivo effects of fluoxetine translate to the in vivo situation (ideally after topical application) and to dissect whether or not topical application of this SSRI also stimulates epidermal hyperpigmentation, which would be undesired in the scalp skin of most individuals undergoing hair greying. In any case, our pilot study strongly encourages systematic exploration of fluoxetine as a candidate hair antigreying agent.
seems like this might relate to the senolytic approaches, and sphingolipids: Senolytics Topically Administered to Skin for Antiaging Effects
Posted that in the IL-17 thread but it’s relevant to that thread too.
[Edit] I just noticed this has been posted before. Sorry about that but the link with the IL-17 discussion makes it more relevant.
Latanoprost, a prostaglandin (PG) F2α analogue, is an effective and widely used medication in the treatment of open-angle glaucoma.1 Since its introduction, several adverse effects have been reported, prominent among which has been increased pigmentation of the iris and eyelashes.2 We describe a patient who developed repigmentation of her previously white hair after latanoprost therapy.
A 65-year-old woman with open-angle glaucoma of 3 years duration mentioned recent changes in her hair colour. She was in a good state of health. Treatment with topical 0.005% latanoprost, 1 drop (∼20 μL) every evening, was started in her both eyes on April 2007. The patient first noticed gradual darkening of her hair, which had been completely white for 20 years, approximately 3 years after she started latanoprost therapy. She referred that her hair had gone progressively greying over the years and she did not report any trigger factors like systemic diseases or stressful events. The repigmentation with the original colour took about 1 year. It started from the root and the proximal part of the hair and then interested all the hair length. She denied the use of any dyeing or new shampoo or any lotion for the scalp during this whole interval. On physical examination, hair darkening was spreading (Fig. 1). Eyebrows and the skin around patient’s eyes were instead unchanged. The patient continued latanoprost therapy and the pigmentation of her hair has persisted after 9 months of follow-up.
The first reported and most prominent adverse effect of latanoprost eye drop is darkening of the irides. More recently reported adverse effects of that drug are hyperpigmentation of the eyelid, neck, back or temporal area of the face2 and also change of eyelashes and vellus hair in the treated eye including pigmentation, increased length, thickness and misdirected growth of eyelashes.1
The underlying mechanism of latanoprost-associated hair and skin change in growth and pigmentation is poorly understood. As latanoprost is a selective agonist for the PGF2α receptor, it is likely that the phenomenon is mediated by this receptor. PG is one of the most potent stimulants of melanogenesis[6] and melanocyte growth7 and the release of PGs, primarily PGE2 and PGF2α, in the skin is one of the mechanisms of action of ultraviolet rays,8the best-known stimulant of melanogenesis.
A study on a guinea pig model has demonstrated that PGF2α analogues (including latanoprost) topically applied increase skin pigmentation.[9]. A study on a murine model has demonstrated the influence of PGF2α and its analogue latanoprost on hair regrowth and follicular melanogenesis.[10]
Preliminary experiments carried out in a human model have shown that a very brief (<21 days) latanoprost exposure cause hypertrichosis of eyelashes that persists also in the absence of the drug.[1]
Although drug-induced repigmentation was the most probable explanation for the hair-colour change in our patient, the relation between drug intake and hair darkening would have been proven only if hair had returned to its original colour after drug withdrawal. We were unable to make such an observation as the patient continued the treatment. Repigmentation of hair is an uncommon phenomenon, and the mechanism continues to defy explanation. We have described a further case illustrating that hair greying is not an irreversible process. In this patient, the repigmentation was imputated to latanoprost therapy, an association not previously reported. Advances in understanding the pathophysiology should promote development of therapeutic approaches to avoid or repair the greying process.
Ask them.
Cheap from the regular India-based suppliers… About $1 to $2 per small bottle…
I wonder if scalp topical use would be a good thing to try with this… mix with transcutol or DMSO…