Rapamycin is the flagship mTOR inhibitor in aging research, with systemic benefits reported in multiple organs. This new study asks a narrower question: can rapamycin help repair an overused Achilles tendon? In a rat model, researchers injected collagenase into the Achilles to mimic tendinopathy, then treated animals with a daily intraperitoneal injection of rapamycin (5 mg/kg). Tendons were followed for 2–8 weeks with histology, immunostaining, Western blotting of the mTOR axis, and 8-week biomechanical testing.
What they found
Collagenase produced a classic degenerative tendon phenotype: disrupted collagen, round-cell infiltration, neovascularization, and progressive chondrometaplasia marked by Col-2 and SOX-9 expression. Rapamycin sharply reduced cell density, improved histologic scores, and almost abolished Col-2/SOX-9 upregulation at 4–8 weeks, indicating suppression of ectopic cartilage formation. Western blot showed that collagenase strongly activated AKT–mTOR–p70/S6K signaling, while rapamycin reversed this phosphorylation, confirming on-target mTOR inhibition and a likely shift toward higher autophagy and lower anabolic drive in the injured tendon.
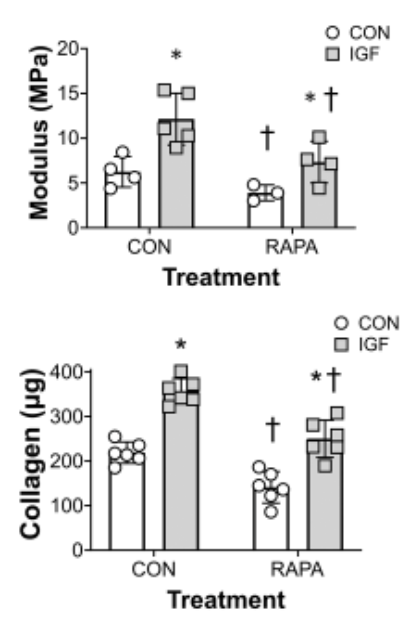
Mechanically, the picture is less encouraging. Rapamycin normalized tendon diameter but did not restore maximum load, tensile stress, or elastic modulus versus collagenase alone; both injured groups remained mechanically weaker than controls at 8 weeks. The authors argue that while mTOR suppression limits pathological chondrogenesis and may optimize collagen architecture, the autophagy–collagen metabolism balance and needle/collagenase damage together constrain strength recovery.
Mechanistic and longevity relevance
For longevity biohackers, this is organ-specific aging in microcosm. Tendons fail with age partly through aberrant matrix remodeling and maladaptive cell fate (tenocytes drifting toward chondrocyte-like states). By dialing down mTOR, rapamycin appears to steer tendon repair away from cartilage-like scarring and toward more tendon-like tissue. Autophagy is implicated but not directly measured; AMPK, cGAS–STING, mitochondrial and vascular endpoints are absent, so any claims about systemic “inflammaging” modulation remain speculative.
What is genuinely novel here is the clear decoupling between histologic “youthfulness” and mechanical function in an Achilles model under explicit mTOR blockade, plus a candid discussion that older animals might show stronger rapamycin responses but were not tested.
Critical Limitations and Translational Uncertainty
- Model mismatch: acute collagenase + needle damage, not chronic overload or age-related degeneration.
- Young animals only; rapamycin as an “anti-aging” drug was not tested in aged tendons.
- High-dose daily IP regimen, unlike intermittent low-dose human longevity protocols.
- Biomechanics not rescued: the single most relevant endpoint for function remains unimproved.
- No direct measures of autophagy flux, mitochondrial quality, cGAS–STING, or vascular remodeling; mechanism is inferred from mTOR phosphorylation rather than fully mapped.
- Follow-up only to 8 weeks; longer-term remodeling unknown.
Further needed: aged-animal models, chronic overload designs, varied rapamycin schedules (including intermittent), direct autophagy/mitochondrial and inflammatory readouts, and human data where tendon structure and function are pre-specified outcomes in rapalog trials.
Full paper (open access) here: Rapamycin-mediated inhibition of the mTOR pathway promotes tendon healing in a collagenase-induced achilles tendinopathy
Disclaimer: All these posts are generated with the help of AI systems, and there could be mistakes. Validate with good medical sources before taking any course of action. This is provided for information purposes only, no medical advice is given here