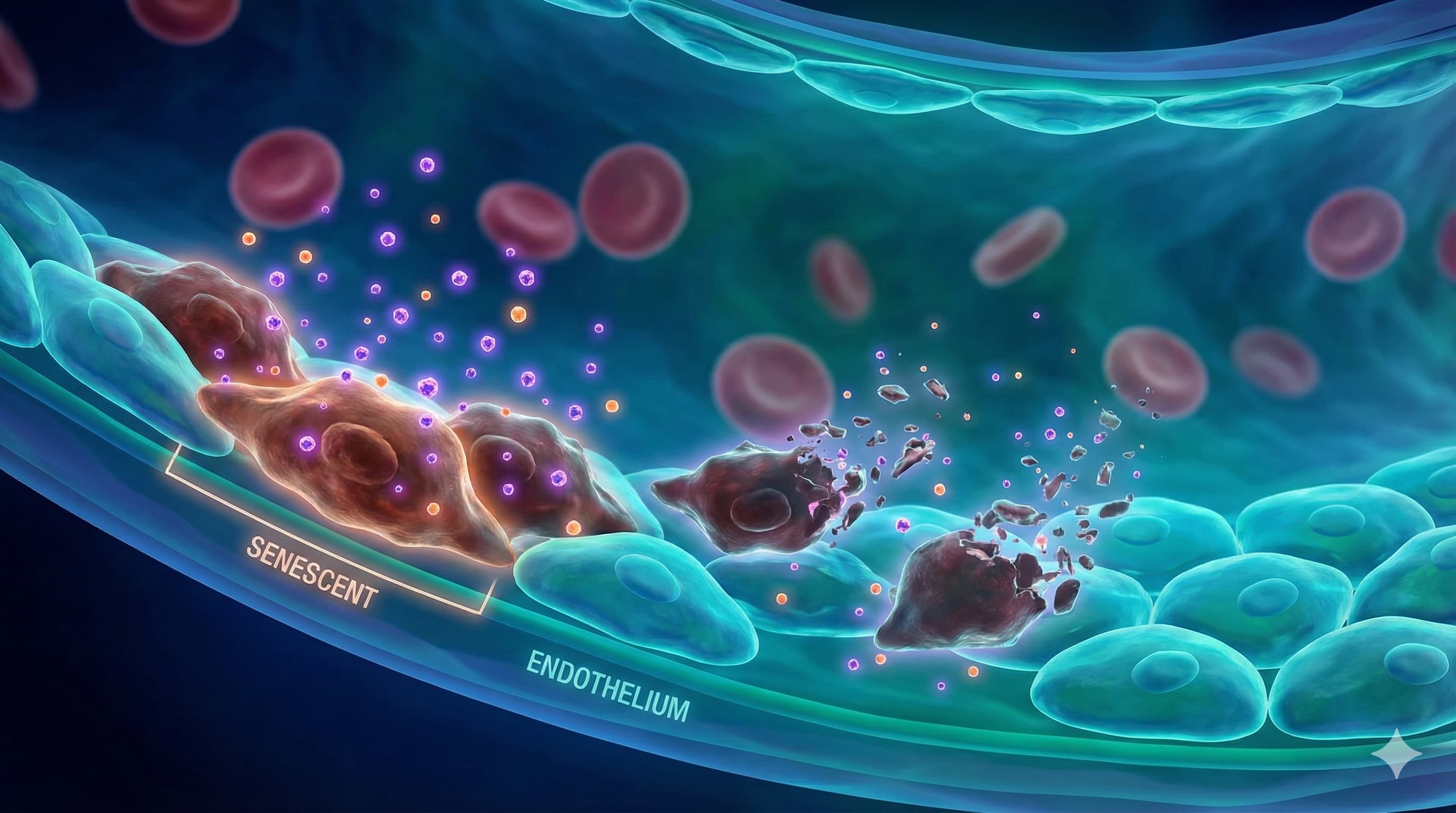
In a significant advancement for vascular longevity, researchers have engineered a “dual-threat” nanotherapeutic that reverses the biological age of blood vessels. By coating selenium nanoparticles (SeNPs) with the longevity drug Rapamycin (RPM), the team created a delivery system that outperforms either compound alone—even when using a fraction of the standard rapamycin dose.
Vascular aging is driven by “senescent” endothelial cells—zombie cells that stop dividing and spew inflammatory toxins, causing arteries to stiffen. This study identifies the breakdown of mitophagy (the recycling of batteries within the cell) as a primary culprit. The new compound, RPM-SeNPs, acts as a precision guided missile. The Rapamycin component inhibits the mTOR pathway to trigger the “cleanup” signal (autophagy), while the Selenium core upregulates GPX4, a critical antioxidant enzyme that protects mitochondria from rusting out (oxidative stress).
The results in mouse models were striking: the nanoparticles cleared senescent cells, restored vessel elasticity, and reduced inflammation markers, all while using approximately one-sixth the dose of free Rapamycin. This suggests that the future of rapamycin therapies may not lie in higher oral doses, but in smarter, targeted delivery systems that dramatically improve safety profiles while amplifying efficacy.
Source:
- Open Access Paper: Rapamycin coated selenium nanoparticles relieve oxidative senescence of vascular endothelium by mitophagy
- Context: Jinan University, China | Journal: Redox Biology
- Impact Evaluation: The impact score of this journal is 11.9 (JIF 2024), Therefore, this is a High Impact journal, ranking in the top quartile (Q1) for biochemistry and redox biology.
Part 2: The Biohacker Analysis
Study Design Specifications
- Type: In vivo (Mouse Model) & In vitro (Mouse Aortic Endothelial Cells - MAECs).
-
Subjects: Male C57BL/6J mice, 6 weeks old.
- Group Size: n = 10 per group.
- Model: Paraquat (PQ) induced oxidative senescence (acute aging model), not natural chronological aging.
- Lifespan Data: None. This is a short-term mechanistic study (14-day protocol).
- Route: Intravenous (IV) injection every other day.
Mechanistic Deep Dive
The study leverages a “One-Two Punch” against cellular aging:
- The Trigger (mTOR Inhibition): The Rapamycin coating suppresses the PI3K/Akt/mTOR pathway. Specifically, it prevents the phosphorylation of ULK1 at Ser757. When ULK1 is not phosphorylated at this site, it is free to initiate mitophagy—the selective digestion of broken mitochondria.
- The Shield (GPX4 Upregulation): Senescent cells are usually terrified of autophagy because their membranes are too oxidized. The Selenium core of the nanoparticle releases selenium that is metabolically converted into GPX4(Glutathione Peroxidase 4).
- Critical Detail: The study emphasizes mitochondrial GPX4 (mtGPX4). By boosting antioxidant capacity inside the mitochondria, the cell becomes healthy enough to undergo recycling without triggering cell death.
Novelty (The “New” Info)
- Micro-Dosing Efficacy: The RPM-SeNPs achieved better anti-senescence results than free Rapamycin despite delivering ~83% less Rapamycin (1/6th the dose). This validates the hypothesis that nanoparticle targeting can radically improve the therapeutic index of longevity drugs.
- Synergy: Free Selenium (SeNPs alone) and Free Rapamycin (RPM alone) worked partially, but the combinationrestored mitochondrial membrane potential and ATP production to near-control levels, suggesting a multiplicative effect.
Critical Limitations
- Short-Term “Stress” Model: The mice were young (6 weeks) and chemically aged with Paraquat. This mimics acute oxidative injury (like reperfusion injury or toxin exposure) rather than the slow, chronic accumulation of senescence seen in natural aging.
- Administration Route: The treatment was Intravenous (IV). SeNPs often have poor oral bioavailability compared to organic selenium (like selenomethionine), and IV translation to human preventive medicine is impractical.
- No Longevity Data: We do not know if these mice live longer. We only know their arteries looked better for a week.
3. Feasibility & ROI
- Availability: Zero. Rapamycin-coated Selenium Nanoparticles are not commercially available.
- DIY Risk: Extreme. Mixing Rapamycin powder with Selenium supplements will not create this nanoparticle. It requires chemical reduction synthesis (using ascorbic acid) to create the core-shell structure. Ingesting high-dose sodium selenite is toxic/lethal.
Part 4: The Strategic FAQ
Q1: How does this compare to current “Longevity” Rapamycin protocols? A: Current protocols (e.g., 6mg weekly) are “blunt instruments” that inhibit mTOR systemically. This study suggests that if we could target the drug, we could use <1% of the dose. This is a glimpse into “Gen 2” longevity tech: Nanomedicine.
Q2: Why use Paraquat to induce aging? A: Paraquat generates massive Reactive Oxygen Species (ROS). It’s a “stress test.” It proves the drug is a potent antioxidant. It does not prove it reverses natural, slow-accumulation aging (chronological aging).
Q3: What is the next step for this technology? A: The authors need to run a Lifespan Study in naturally aging mice. If these nanoparticles extend life with such micro-doses of Rapamycin, it would revolutionize the field. Until then, it is a promising vascular therapy concept.