Not just cheap, but very convenient. If normobaric.
Bryan Johnson stops methylene blue, arguing that MB “is making [him] less tolerant of hypoxia”.
On mtDNA and hypoxia @John_Hemming:
- Long-term cycles of hypoxia and normoxia increase the contents of liver mitochondrial DNA in rats 2012: “normoxia (group I), or 5,000 m (barometric pressure about 405.35 mmHg) above the sea level (a hypoxic condition) for 23 and 1 h normoxia daily for five consecutive days (group II), 15 days (group III), and 30 days (group IV)” (weirdly, the abstract contains an inconsistency: does long-term increase or decrease mtDNA?)
- Altitude can alter the mtDNA copy number and nDNA integrity in sperm 2011: “Sperm mtDNA copy number for those living at high altitude (5,300 m) for one month was significantly higher (P < 0.05) than for those at the lower altitude (1,400 m) or in donors who had been living at the 5,300 m altitude for 1 year. In addition, sperm mtDNA copy numbers were remarkably decreased (P < 0.05) in those who had lived at the greater altitude for 1 year compared to those who had lived there for one month. The ratio of nDNA integrity among the 10,000 sperms at high altitude for one month was significantly lower (P < 0.05) than that at the lower altitude (1,400 m) or at 5,300 m for 1 year, and the ratio of nDNA integrity sperms at high altitude for 1 year was increased, and higher than that for at the lower altitude (P < 0.05).”
- Analysis of mitochondrial DNA sequence and copy number variation across five high-altitude species and their low-altitude relatives 2018: “Higher mitochondrial DNA control region’s genetic diversity is conspicuous in high-altitude animals versus low-altitude relatives. We also found an accordant decrease of mtDNA copy number in most of the tissues from high-altitude animals.”
- The Dynamic Genome at Altitude: Mitochondrial DNA Copy Numbers in Acute and Chronic Hypoxia 2024: very interesting one comparing populations AND altitude
- Intermittent Hypoxic Conditioning Rescues Cognition and Mitochondrial Bioenergetic Profile in the Triple Transgenic Mouse Model of Alzheimer’s Disease 2021: “IHC caused a significant increase in brain cortical levels of glucose and a robust improvement of the mitochondrial bioenergetic profile in 3×Tg-AD mice, as mirrored by the significant increase in mitochondrial membrane potential (ΔΨm) […] IHC was able to induce a significant increment in the relative mtDNA copy number in the brain cortex of 3×Tg-AD mice”
One more: Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans 2001
The present study investigated the effect of a single bout of exhaustive exercise on the generation of DNA strand breaks and oxidative DNA damage under normal conditions and at high-altitude hypoxia (4559 meters for 3 days).
4,559 m is about 11.5% FiO2
That’s probably got NF kappa B going.
Interesting chart + reasoning @John_Hemming: Health benefits of life at moderate altitude: does hypoxia matter? 2025
Epidemiological studies show that living at moderate altitudes, i.e., 1,000–2,500 m, is associated with beneficial health effects when compared to lower altitudes. […] However, it remains unclear how the small decrease in barometric – and associated partial oxygen – pressure at moderate altitude could provoke beneficial adaptive responses. At least in awake and resting humans, the resulting mild hypoxic conditions are not thought to induce robust hypoxic responses. This opinion article discusses why hypoxic episodes can occur even at moderate altitudes and which effects they may trigger to contribute to the beneficial health outcomes mentioned above.
The oxygen-hemoglobin dissociation curve (ODC, Figure 1) illustrates that there is an apparently negligible decline of the arterial oxygen saturation (SaO2) when moving from sea level to moderate altitudes, i.e., 1,000–2,500 m, in the provided example in Figure 1 of <2%. This is the case despite an expected large decrease of the arterial partial pressure of oxygen (PaO2) from about 100 mmHg to 70 mmHg at 2,240 m (Cid-Juárez et al., 2023). From this point (the knee of the ODC), a steep decline of SaO2 follows with each further drop in PaO2.
Depending on factors like increased temperature and decreased pH of blood, the ODC is shifted to the right during physical exercise. This results in a much more pronounced (>6%) drop in SaO2 already at moderate altitude (i.e., 2,240 m in the example of Figure 1) compared to sea level conditions. Thus, during exercise, SaO2 drops from about 95% at rest to below 90%, usually considered to indicate hypoxemia. Similarly, PaO2 also declines during mild hypoventilation (e.g., during normal sleep or sleep-disordered breathing), resulting in considerably more pronounced decreases of SaO2 at moderate altitudes than at sea level (Rojas-Córdova et al., 2023). This behavior of the ODC may be highly relevant for the more than 860 million people residing at moderate altitudes between 1,000 and 2,500 m worldwide (Tremblay and Ainslie, 2021) and also for the many millions of people annually visiting moderate altitudes for sightseeing, hiking and skiing (Burtscher et al., 2023b). Consequently, people living at or sojourning to moderate altitudes repeatedly experience physiologically relevant drops in oxygen availability, likely inducing a phenomenon similar to “hypoxia conditioning”.
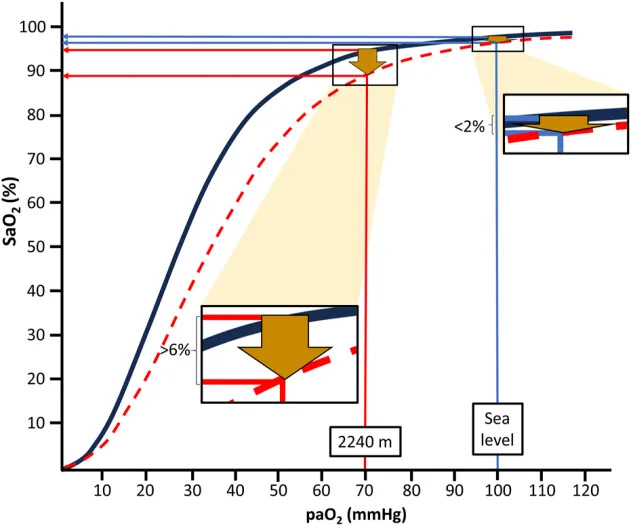
=> So what is the SpO2 and paO2 of someone living at 2,000m of altitute during sleep or exercise?
Mortality was reduced in both sexes when living between 1,000 and 2,000 m compared to those living lower: by 15% (13–18%) in men and by 22% (20–24%) in women (p < 0.05).
Also Cross-posting this n=2 trial in PD: Parkinson's disease - #933 by adssx
You and I have worked this one out. The HIF response is from a change in PaO2, but there is a benefit from lower chronic PaO2 which arises from lower ROS.
Yes but I also think that:
- There might be another HIF-independent effect of intermittent hypoxia (based on worms and mice studies).
- Hypoxia conditioning via intermittent hypoxic exposure might be beneficial long-term to restore a correct hypoxic response.
I think you may be right.
Sexual dimorphisms in phrenic long-term facilitation following severe acute intermittent hypoxia 2025
It’s a preprint showing different effects in male vs female rats:
Rigorous pre-clinical research in male rodents defined the cellular mechanisms of respiratory neuroplasticity following brief exposures to hypoxia (acute, intermittent hypoxia; AIH). AIH elicits phrenic long-term facilitation (pLTF), a progressive increase in phrenic nerve amplitude over time. Mechanisms to AIH-induced pLTF are complex and variable depending on the severity of hypoxemia during AIH. Moderate AIH (mAIH; PaO2 ~35-45mmHg) triggers spinal serotonin receptor activation to induce pLTF expression. More severe AIH (sAIH; PaO2 ~25-30mmHg) induces pLTF through an adenosine receptor-dependent pathway. Here we assessed: 1) if sAIH-induced pLTF is expressed in female rats, and whether sAIH-pLTF is impacted by the estrous cycle; 2) if the magnitude of sAIH-induced pLTF in female rats is similar to male rats; and 3) whether GDX alters the magnitude of sAIH-induced pLTF. We hypothesized that female rats would express sAIH-induced pLTF, and that circulating steroid hormone levels would have minimal impact on sAIH-induced pLTF in either sex. Our findings reveal that female rats express robust pLTF (~106% above baseline phrenic amplitudes) in response to sAIH, with minimal effects of estrous cycle stage. Female rats also showed a nearly 50% higher magnitude in sAIH-pLTF than males (p=0.006). Following GDX, pLTF magnitude was reduced in female rats (p=0.04), while males were unable to express pLTF. These findings predict unique cellular mechanisms to pLTF in female rats following sAIH, and sex-specific impacts of steroid hormone signaling on the expression of respiratory neuroplasticity.
They conclude:
Trumbower and colleagues demonstrated this idea by showing that caffeine, a common A2A receptor antagonist, augmented AIH-induced gains in walking function in individuals with chronic spinal cord injury. Should there be unique, female-specific mechanisms to sAIH, then the chosen targets to enhance the clinical effects of AIH in women may be different.
To ascertain the effect of hypoxic/hyperoxic shifts on immunity, a series of 11 days hypobaric chamber studies were conducted at the Johnson Space Center. The living environment consisted of 8.2–9.6psi/28.5%–34% oxygen, and there were several simulated EVAs which were performed under hypobaric/hyperoxic conditions consisting of 4.3psi/85%–95% oxygen. For the current sub-study, biosamples were collected before and after simulated EVAs to ascertain the effects of hypoxia, decompression and hyperoxic stress on immunity. The sub-study consisted of 3 chamber tests, 23 total subjects.
The primary finding from this study is that, while living in a mildly hypoxic exploration atmosphere did not have a profound impact on immunity, the participation in hypobaric/hyperoxic EVA activities impacted numerous and varied immune parameters.
They used very mild hypoxia equivalent to 18-19% FiO2.
Effect of Repeated-Sprint Training in Hypoxia in Female National-Level Rugby Union Players 2025
Purpose: The aim of this study was to investigate the effects of repeated-sprint training in hypoxia (RSH) versus in normoxia (RSN) in female national-level rugby union players.
Methods: In a randomized, controlled, and crossover study, 8 female rugby union players performed 5 sessions of repeated sprints either in normobaric hypoxia (RSH, simulated altitude: 3000 m; FiO2 = 14.5%) or in normoxia (RSN, terrestrial altitude: 165 m; FiO2 = 20.5%). Before (Pre) and after (Post) training, repeated-sprint ability (6 × 10-s “all-out” sprints and 20-s recovery) was evaluated on a cycle ergometer.
Results: From Pre to Post, peak power output was improved in RSH (602 [98] vs 704 [92] W; P = .007) but not in RSN (661 [91] vs 673 [76] W; P = .560). Similarly, mean power output was enhanced in RSH (445 [63] vs 532 [51] W; P = .013) but not in RSN (499 [88] vs 509 [63] W; P = .557). Sprint decrement did not change in either RSH (24.5 [8.9] vs. 24.0% [5.7%]; P = .819) or RSN (22.7 [5.9] vs 24.3% [4.8%]; P = .336).
Conclusion: As few as 5 sessions of RSH were beneficial for improving peak and mean power outputs during repeated-sprint exercise in female national-level rugby union players compared with the same training in normoxia.
May be due to reduction in serotonin in certain areas of the brain:
Hypoxia can adversely affect multiple organ systems. This study investigated the impact of intermittent hypoxia on serotonin levels and depression-like behaviors across distinct neuroanatomical regions. Sixteen adult female Wistar albino rats were divided into two groups: control (n = 8) and hypoxia (n = 8). The hypoxia group was exposed to a simulated altitude of 3000 for 5 h daily over 14 days. Behavioral assessments included locomotor activity (open field test) and depression-like behaviors (forced swimming test). Serotonin levels were quantified via ELISA in the prefrontal cortex, striatum, thalamus, hypothalamus, hippocampus, and serum. Intermittent hypoxia did not alter locomotor activity (p > 0.05) but significantly increased depression-like behavior (p < 0.05), accompanied by a pronounced reduction in swimming behavior (p < 0.0001), a marker associated with serotonergic function. Serotonin levels were significantly reduced in the prefrontal cortex (p < 0.005) and striatum (p < 0.05), while no changes were observed in other regions or serum (p > 0.05). These findings demonstrate that intermittent hypoxia induces depression-like behaviors and region-specific serotonin depletion, particularly in the prefrontal cortex and striatum. This underscores the need to evaluate hypoxia-related brain health implications in conditions such as sleep apnea and acute mountain sickness.
Canadian preprint: Resynchronization of the biological clock using exposure to low oxygen levels in humans: an exploratory study 2025
In condition 1 (Hypo), participants underwent a 2-hour normobaric hypoxic exposure (FiO2 = 12%), starting 2 h after habitual wake time. In condition 2 (Lum+Mel), participants received a 3-hour luminotherapy session (500 nm, 506 lux) at the same time point, combined with 5 mg of exogenous melatonin administered 6 hours before usual bedtime. Salivary melatonin levels were measured in each phase of the study to assess circadian phase shifts.
Salivary melatonin levels increased progressively over time in all conditions (p < 0.001), with significant differences observed between experimental conditions (p < 0.001), but no interaction effect (p = 0.854). Exposure to hypoxia significantly reduced oxyhemoglobin saturation (p < 0.05) and increased heart rate and subjective symptoms of fatigue. In terms of circadian phase, the dim light melatonin onset (DLMO) occurred 1.30 hour (78 minutes) earlier in the Lum+Mel condition compared to baseline (p=0.001). In the hypoxia condition, the DLMO occurred on average 0.58 hour (34.8 minutes) earlier than baseline, but this change did not reach statistical significance (p=0.156).
This study provides preliminary evidence that normobaric hypoxia may modestly advance the human circadian phase, although not to a statistically significant extent. In contrast, combined phototherapy and melatonin administration produced a robust and significant phase advance in salivary melatonin onset. These findings suggest that while hypoxia may influence circadian timing, established interventions like light and melatonin remain more effective for circadian realignment. Further research is warranted to elucidate the mechanisms and optimize the application of hypoxia in circadian modulation.
If hypoxia can increase melatonin secretion that’s interesting @John_Hemming.
Its an interesting thought.
Hypoxic conditioning in Parkinson’s disease: randomized controlled multiple N-of-1 trials 2025
It’s been a long wait… Results of the phase 1 trial of hypoxia in PD. tl;dr:
- It’s safe (based on subjective feelings and brain biomarkers)
- Intermittent hypoxia is better than continuous
- Small symptomatic improvements
- 16.3% FiO2 is better than 12.7%
Based on these results, they ran a phase 2 trial at 16.3% over several weeks. Results due to be published in the next 12 months…
“These protocols typically consist of repeated 4–8 min exposures to hypoxia, i.e. fractions of inspired oxygen (FIO 2 = 10–15%, alternating with 2–5 min exposures to normoxia (IHE: FIO 2 = 21%) or hyperoxia (IHHE: FIO 2 = 30–35%), totalling 35–40 min/session applied 3–5 times per week for 3–8 weeks. IHHE has also been termed intermittent hypoxia hyperoxic conditioning (IHHC)”
I’m curious if anyone here has been using this protocol and taking Rapamycin. I’ve been doing it 3-4 Xs a week going between a hypoxicator and oxygen concentrator since reading the book [Undoing Lyme Disease].
Also, cycling on and off once a week Rapamycin.
3 sessions per week over an 8-week period
Sessions began with a 5-min baseline period while breathing room air, followed by seven cycles of hypoxia, each lasting 5 min interspersed with 3-min periods of normoxic breathing (Figure 2). Using a gas-mixing device (Altitrainer®, SMTEC S.A., Nyon, Switzerland), the inspired fraction of oxygen (O2) was individually adjusted during each session to achieve a target arterial O2 saturation assessed by pulse oximetry (SpO2 = 80% from week 1 to week 4; 75% from week 5 to week 8) (Radical-7®, Massimo corporation, Irvine, CA, USA). Each session ended with a 5-min normoxic phase, allowing participants to return to their pre-session physiological state. In total, each session lasted 63 min, including 35 min of hypoxic breathing.
Brachial artery FMD, cardiopulmonary exercise testing (CPET), and ambulatory 24-h blood pressure were assessed at baseline (Pre), immediately post-intervention (Post 1), and 2 months later (Post 2). FMD showed a trend toward improvement in the IHC group, being significant when normalized for baseline artery diameter (p = 0.023; ηp2 = 0.150) between Pre and Post 2. Peak ventilation during CPET increased from Pre to Post 1 (p = 0.021), with no other significant CRF changes. Daytime systolic blood pressure decreased by 6 mmHg (p = 0.070, ηp2 = 0.105). No significant alterations in these outcomes were observed in the CTL group (p > 0.05). Moderate IHC enhanced mid-term endothelial function, suggesting potential to mitigate age-related vascular decline.
Healthy young adults (N = 24) completed an IH protocol entailing 12 alternating 5-min normoxic (PETO2 = 100 mmHg) and hypoxic (PETO2 = 50 mmHg) intervals that were normocapnic and isocapnic, and on a separate day completed a time-matched normoxic control protocol. Prior to (T0), and immediately (T1) and 30 min (T2) following each protocol, EF was assessed via the antisaccade task. Antisaccades require a goal-directed eye movement (i.e., saccade) mirror-symmetrical to a target and provide the resolution to detect subtle EF changes. As expected, hypoxic intervals decreased arterial and cerebral tissue O2 saturation and increased CBF as estimated via near-infrared spectroscopy and transcranial Doppler ultrasound (ps < 0.001). In turn, antisaccade reaction times (RT) did not differ between T0 and T1 (p = 0.29); however, at T2 a reliable RT reduction was observed (p = 0.004).
Better reaction time after intermittent hypoxia @John_Hemming . They used approximately 10% FiO2! On the other hand that other paper did not find cognitive benefits:
Twelve volunteers with chronic TBI underwent four AIH sessions conducted on separate days, in which they were exposed to fifteen 30-60-s hypoxic episodes interspersed with 60-90 s of breathing ambient air. Inspired oxygen (O2) concentration during hypoxic episodes was gradually reduced from 21% (equal to ambient air, sham), to 17%, 13%, and 9%, over the course of four sessions.
No significant improvement in cognition or mood was noted after the AIH intervention.
Motor performance gradually improved over the course of the study, but no significant changes in response to TMS were found in corticospinal excitability.
AIH dosage as low as 9% O2 appears safe to use in chronic TBI, but its potential benefits remain to be investigated.
Intermittent hypoxia promotes fatty acid metabolism and improves myocardial energy homeostasis potentially via AMPKα1 pathway 2025: “IH intervention enhances fatty acid metabolism, improves mitochondrial tricarboxylic acid cycle efficiency, promotes ATP synthesis, preserves mitochondrial ultrastructure, reduces fibrosis, and improves cardiac function in MI rats, potentially through AMPKα1 pathway activation.”
I wonder if there is a differential impact of hypoxia treatment on those who permanently reside at high elevations.