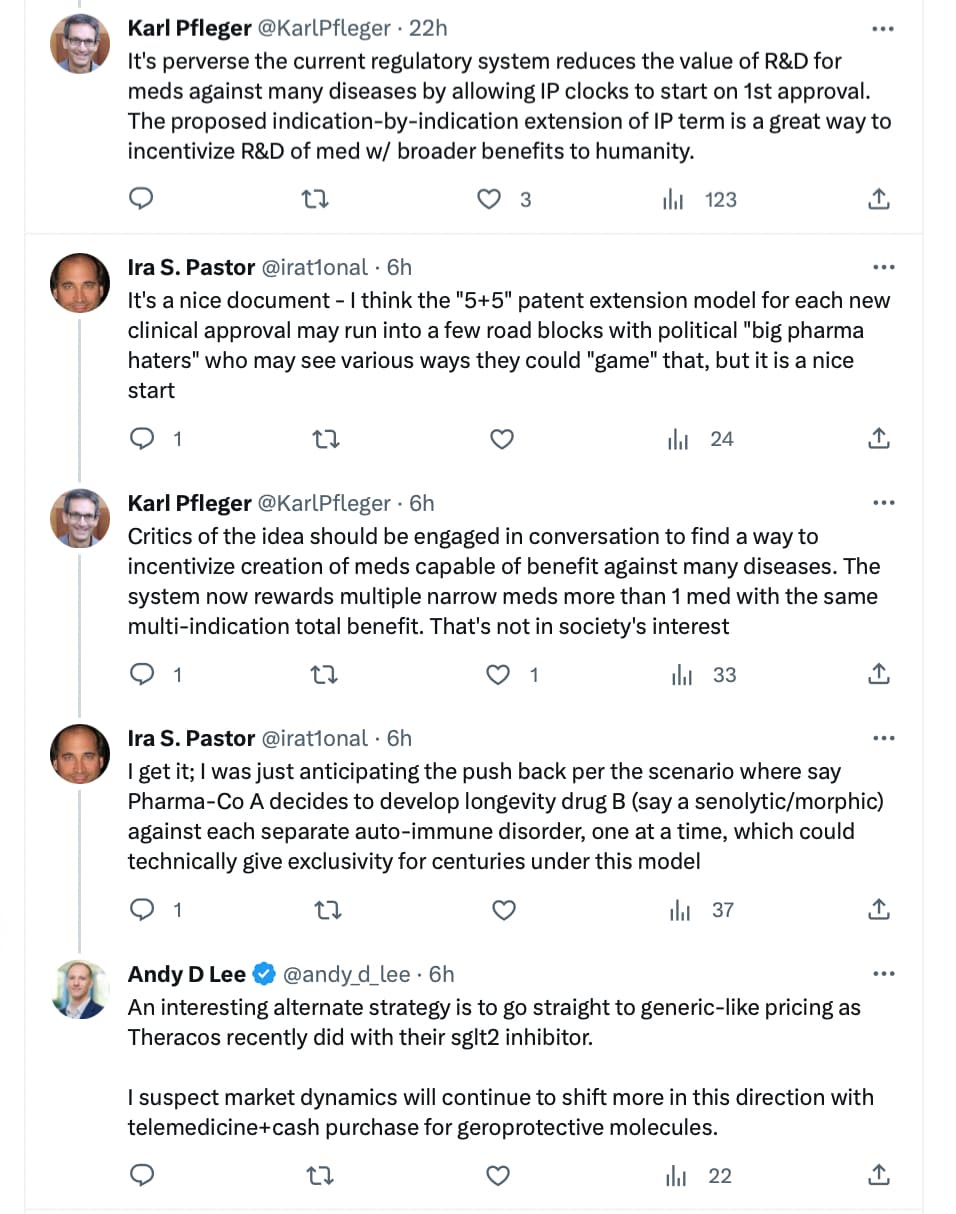
The white paper: The Advanced Approval Pathway for Longevity Medicines is an overview of how this non-profit longevity organization proposes to increase the flow of longevity drugs to market. 15 pages but well worth a read.
More people may want to read it & sign the petition (see below) and the organization’s other petitions. Please spread the word on all the social media platforms.
They also have a petition to Increase appropriations to the National Institute on Aging - Division of Aging Biology; the group the funds Richard Miller’s excellent ITP program that tests potential longevity medications in a rigorous 3-site program. I highly recommend signing that petition as I think the NIA ITP program has done more than anyone in the world to move longevity medicine forward (and their current budget is under $5 Million / year).
To sign their petitions, click on the link below titled “Petitions” and see how you can help:
Full Whitepaper below:
AAPLM-Whitepaper-Rewrite1.pdf (384.9 KB)