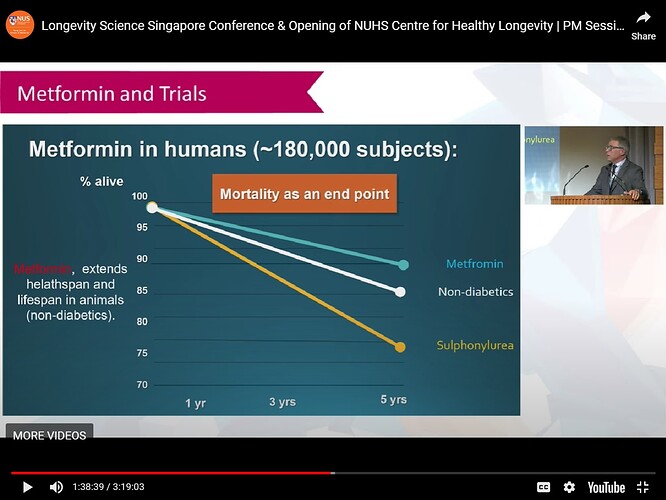
First of all, most of the mouse models of diseases are very specifically genetically modified mice that are changed in a way that “estimates” a disease or disease process. A great example of this are the Alzheimer mouse models. If the “estimate” of the genetic changes made to look like a disease is wrong, then the treatment won’t work in humans.
Most of the mouse models are not very good models for the diseases they are trying to mimic. See more here as an example of this problem: The trouble with mice as behavioral models for Alzheimer's | STAT
In biology of aging research the situation is vastly different; nobody is creating a special mouse model of aging. These are regular (within a range of specified sources) mice, doing regular aging. So - the “aging model” has to be accurate, because people aren’t genetically modifying the mice to seem to be aging. And, we know that aging in mice looks a lot like aging in dogs and aging in humans.
See Richard Miller’s (of the NIA ITP Program) response to this very question in an interview by a Lifespan IO person:
Lifespan IO: Yes, we’ve had a lot of success in mice, but many drugs that work in mice do not work in humans.
Richard Miller: And many drugs that work in mice do work in humans. It would be silly to maintain that the percentage that work is zero, and it would be equally silly to maintain the percentage that fail is zero.
Most of the drugs that were developed for therapeutic effect in people were initially discovered by working on mice and rats. It would be nuts to say that every drug that extends lifespan in mice will do the same thing in humans, but the work in mice is a very important foundation.
Many of the pathways that are discovered, and maybe even some of the druggable targets that are first discovered, in the mice will serve in humans – maybe the same drug, maybe drugs of the same family, maybe drugs that target the same molecule, but through a different chemical grouping. It’s necessary to be neither insanely optimistic nor insanely pessimistic.
Lifespan IO: We do have a history of failures though, such as with Alzheimer’s, maybe because mice don’t really develop Alzheimer’s.
Richard Miller: Yes, that’s true, but it’s important to recognize the brains of people and the brains of mice have a lot of things that are not in common. In terms of aging, if I tell you that I have an individual right here in front of me, in my office, that has cataracts, bad hearing, weakened bones, a poor immune system, and a relatively low cardiovascular system, you would immediately recognize that individual as old, be it a mouse, a dog, a horse, or a person. But you wouldn’t know if that’s a seventy-year-old human, or a 25-year-old horse, or a three-year-old mouse.
So, the effects that aging has on mice and on humans are – not in every case, of course, but in most cases – recognizably quite similar. And that’s true for cells that divide, for cells that don’t divide, for structures like the bones and the tendons that are mostly extracellular material. It’s true for complicated circuits, like neuroendocrine feedback circuits, it’s true for cognition.
There are just so many aspects – not all, but so many aspects of aging in humans, mice, dogs, chimps, et cetera that are the same. So, it’s very reasonable to expect that the drug that could block aging effects in all of those tissues in mice might also do very similar things in people.
Lifespan IO: But different species die in old age for different reasons. For instance, around 80% of lab mice die of cancer, I think.
Richard Miller: The specific thing that kills the animal is of secondary importance when you’re studying the biology of aging. For instance, elephants die because their teeth wear down and they can no longer eat. When they’re 60 or 70, they have lost their last set of molars and they can’t chew food anymore. Mice, at least those that are used in aging research, indeed die mostly of tumors. People that eat a lot of fatty foods and watch TV, die mostly of atherosclerosis. In people that were alive a hundred thousand years ago, the most prevalent cause of death was probably breaking a bone and not being able to keep up with the group.
The point is not what is the specific cause of death in a specific environmental setting and in a specific species. The real question is what is it that postpones age-associated decline in bones, brains, the immune system, the sensory systems, the gut, and everything else for many decades in people, for a few years in mice, and for 20 years in horses. The factors that regulate the timing of the aging process, I would guess, is very similar in nearly all kinds of mammals.
Source:
Also - related, see this video (first 5 minutes):
“MTOR is a protein kinase that is an evolutionarily conserved mechanism that regulates aging. So, in every species studied to date, whether you inhibit MTOR genetically or pharmacologically, you increase lifespan and healthspan…”