The Google Gemini Deep Research Summary and Analysis of the presentation and related papers, etc.:
Comprehensive Research Report: Critical Analysis of Galectin-3 Inhibition, TB006, and Multimodal Strategies in the Treatment of Alzheimer’s Disease and Related Disorders
1. Introduction: The Paradigm Shift in Neurodegenerative Therapeutics
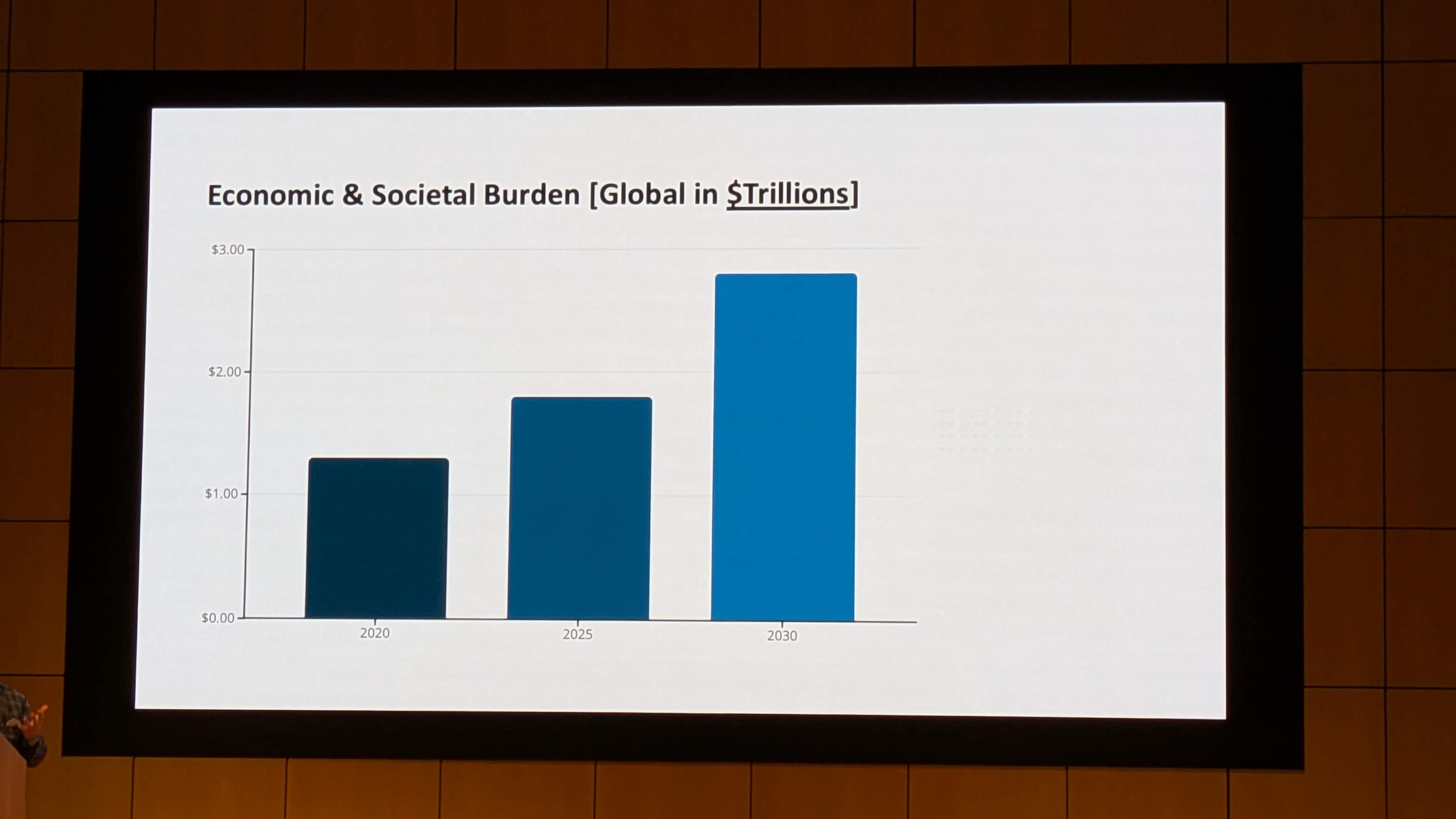
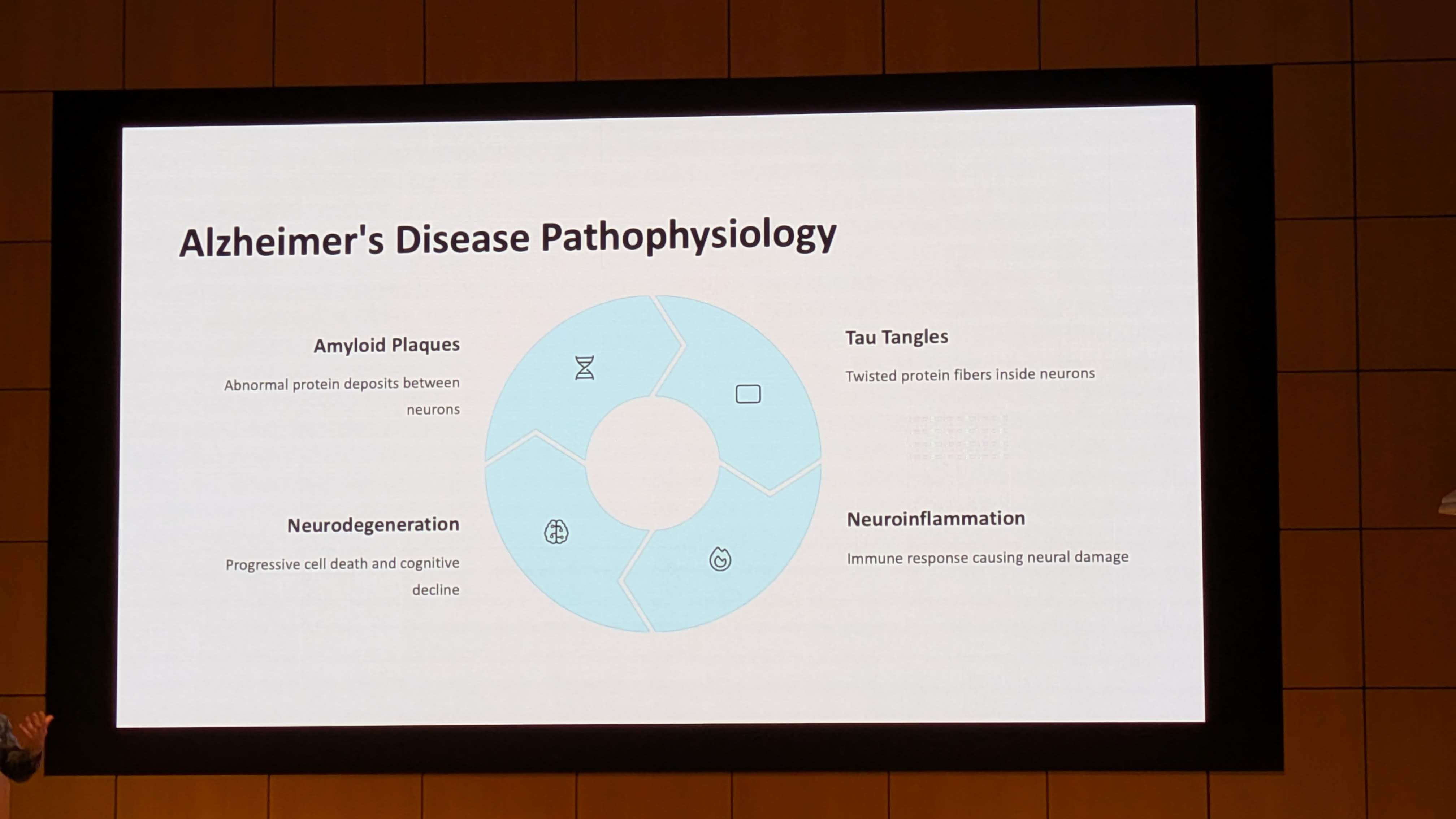
The clinical landscape of Alzheimer’s disease (AD) is currently undergoing a seismic shift. For nearly three decades, the “Amyloid Cascade Hypothesis” has dominated research, guiding billions of dollars into the development of monoclonal antibodies targeting amyloid-beta (Aβ) plaques. While recent approvals of agents like lecanemab and donanemab have validated the ability to clear plaques, the clinical benefits have been statistically significant yet functionally modest, typically slowing cognitive decline by 27% to 35% over 18 months. Crucially, these therapies do not stop the disease, nor do they reverse it, and they carry significant risks of Amyloid-Related Imaging Abnormalities (ARIA), including cerebral edema and hemorrhage.
Against this backdrop, the presentation by Dr. Joshua Helman regarding TB006 (TrueBinding, Inc.) and the targeting of Galectin-3 (Gal-3) represents a divergent and potentially transformative therapeutic avenue. This report provides an exhaustive analysis of the scientific discoveries presented, the mechanistic rationale of TB006, the clinical data supporting claims of “disease reversal,” and the broader context of lifestyle medicine as a validated intervention.
This analysis integrates emerging research on Low-Complexity Domains (LCDs)—highlighted by the 2025 Lasker Basic Medical Research Award—and critically evaluates the intersection of pharmaceutical intervention with the multimodal lifestyle protocols advocated by Dr. Helman and validated by the 2024 Dean Ornish study.
1.2 The Scope of This Report
This report is structured to provide a granular analysis of the following vectors:
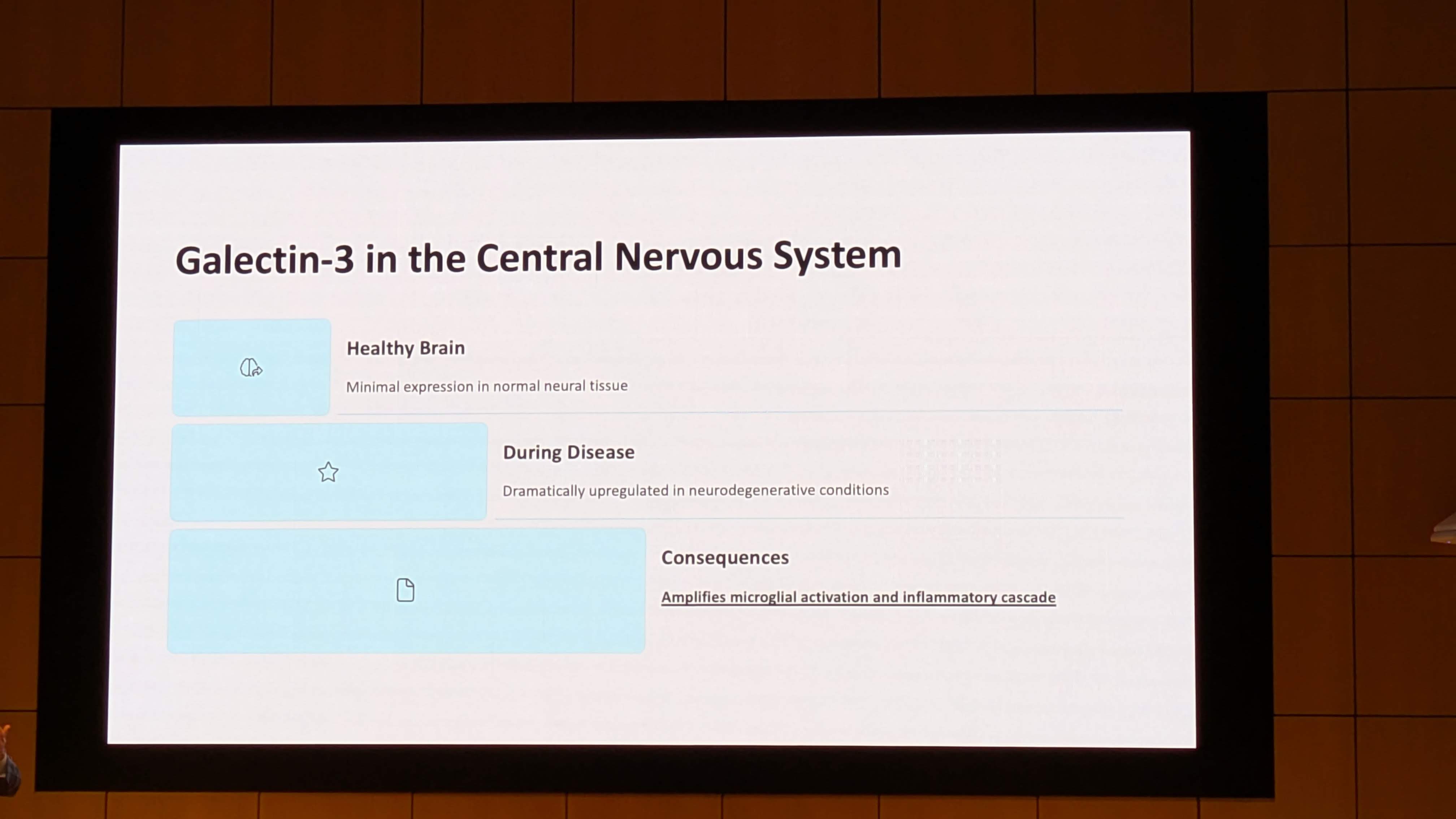
- Molecular Biology: The structure of Galectin-3, its role in Liquid-Liquid Phase Separation (LLPS), and its connection to the 2025 Lasker Award-winning research on Low-Complexity Domains.
- Clinical Efficacy: A deep dive into the TB006 Phase 1b/2a clinical trial (NCT05074498) and the Open-Label Extension (NCT05476783), scrutinizing the statistical significance (p=0.08) versus the clinical magnitude of effect.
- The Helman Protocol: An examination of Dr. Joshua Helman’s “200 Root Causes” theory, evaluating the role of toxins, infections, and lifestyle factors in potentiating Gal-3 pathology.
- Integrative Validation: An analysis of the 2024 Dean Ornish study on lifestyle intervention, providing independent validation for the non-pharmacological components of the proposed treatment protocols.
- Regulatory & Economic Implications: A critical look at the Expanded Access Program (EAP), the cost barriers to access, and the ethical dimensions of patient-funded research.
The Expanded Access Program (EAP)
Because TB006 is not FDA-approved, it is available only through clinical trials or an Expanded Access Program (NCT05959239).
- Structure: EAP allows patients with serious diseases to access investigational drugs outside of trials when no comparable therapy exists.
-
Cost: Unlike trials, EAP is patient-funded. TrueBinding charges for the “manufacturing and administrative” costs.
- Price Tag: Estimates place the cost at ~$5,000 per infusion. With a monthly dosing schedule, the annual cost exceeds $60,000.
- Insurance: Commercial insurance and Medicare do not cover EAP costs.
See full Gemini Deep Research Report here: https://gemini.google.com/share/8b6ee7410b93
1 Like
Interesting notes but I can’t stomach the big block of AI digest.
Only here to mention that PectaSol-C already crushes Galectin-3 and it’s basically a good tasting — albeit expensive— fiber that chelates lead and mercury to boot so why not take that instead of some new experimental drug?
6 Likes
Interesting… thanks for posting this.
Here is what Gemini Deep Research says on this:
Limitations of PectaSol-C
- Bioavailability Variance: As an orally administered complex carbohydrate, the absorption of PectaSol-C is subject to significant inter-individual variability. Factors such as gastric pH, gut motility, and microbiome composition can alter the effective dose absorbed into the blood.
- Lower Affinity: The interaction between a carbohydrate and a lectin is inherently weaker (micromolar range) than an antibody-antigen interaction (nanomolar/picomolar range). To achieve the same level of inhibition as TB006, PectaSol-C relies on “mass action”—flooding the system with inhibitor. In areas of intense inflammation with high local Gal-3 concentrations, PectaSol-C might be outcompeted.
- Dosing Burden: Achieving the necessary serum concentration often requires the ingestion of significant amounts of powder (e.g., 15-30 grams per day). This high volume of fiber can lead to gastrointestinal distress (bloating, gas, loose stools), which may affect patient compliance.
PectaSol-C is best conceptualized as a foundational maintenance agent. Its safety profile, oral delivery, and pleiotropic benefits (detoxification, gut health, cardiovascular support) make it ideal for:
- Prophylaxis: Early intervention in MCI (Mild Cognitive Impairment) before extensive damage occurs.
- Adjuvant Therapy: Supporting antibody therapy by managing the background systemic inflammation and gut health, potentially allowing for lower or less frequent dosing of the biologic.
- Maintenance: Long-term management of Gal-3 levels after an initial course of antibody therapy.
Economic and Logistical Considerations
From a healthcare systems perspective, TB006 faces the high hurdles of biologic pricing and administration logistics. PectaSol-C, being a scalable nutraceutical, offers a “low-hanging fruit” for population-level risk reduction. However, the lack of large-scale, pharmaceutical-grade randomized control trials (RCTs) for PectaSol-C in AD (compared to the rigorous regulatory pathway TB006 is likely undergoing) remains a barrier to widespread clinical adoption by neurologists.
Full analysis here: https://gemini.google.com/share/a260ac99aef0
1 Like
I’ll only say that I’ve taken 15 g of PectaSol a day with no problem at all. Zero GI distress. It was recommended to start off chelation protocols with a loading dose of 15-20 g or more for the first few days, split into chunks every few hours. I stopped taking it simply because I had a fear that it might mobilize any lead or heavy metals which might then go into my milk and my toddler shows no signs of wanting to wean. But the equivalent dose is very easy for me to achieve.
3 Likes
FWIW: from ChatGPT 5
Common Gal-3 inhibitors (examples)
| Category | Examples |
|---|---|
| Modified polysaccharides | Modified citrus pectin (MCP) |
| Small-molecule inhibitors | TD139 (GB0139 – pulmonary fibrosis drug) |
| Natural polyphenols (weak/modest) | Quercetin, luteolin, resveratrol |
| Experimental agents | Belapectin (GR-MD-02) |
2 Likes
That’s what PectaSol is. It’s the easiest way to go — anti fibrosis drugs are expensive, hard to get ahold of, and have side effects. And those other natural compounds are too weak. Experimental drugs obviously unknown unknowns and no access.
1 Like
This is amazing stuff. Blocking galectin 3 is huge for cancer, heart disease and now alzheimers. I take pectasol daily also because I want to get rid of the heavy metals, but if this completely blocks it I think it could be a great thing for longevity. Great tool.
2 Likes
Even that is essentially a modified pectin — note the name. After researching it it sounds like they wanted another pectin that could be trademarked since PectaSol is not sold as a drug. But it should fall under the first heading of modified polysaccharides.
1 Like
There doesn’t seem to any strong evidence that Pectasol actually lowers galectin-3 in humans. AI can’t seem to find anything except this study which essentially showed no significant improvement “In a randomized placebo-controlled trial of MCP, Gal-3 inhibition did not influence collagen markers, echocardiographic measures, or vascular function.”
I just got galectin-3 measured. I will give it a try anyway and measure again in month. Has anyone had a measured reduction in their galectin-3 after starting Pectasol?
1 Like
Yes - that seems to be an accurate statement. I just did a “Deep Search” gemini analysis on exactly this issue and here is the result:
5. Human Clinical Evidence: The Verdict on Lowering vs. Blocking
This section analyzes the pivotal human trials. The contrast between the animal data (strong “lowering” signal) and human data (mixed/negative “lowering” signal) is the most critical insight for the user.
5.1 The Hypertension & Fibrosis Trial (Lau et al., 2021)
This study represents the most rigorous, independent attempt to translate the anti-fibrotic hypothesis of MCP into humans.
- Study Context: Conducted by researchers at Massachusetts General Hospital, this was a randomized, double-blind, placebo-controlled trial.
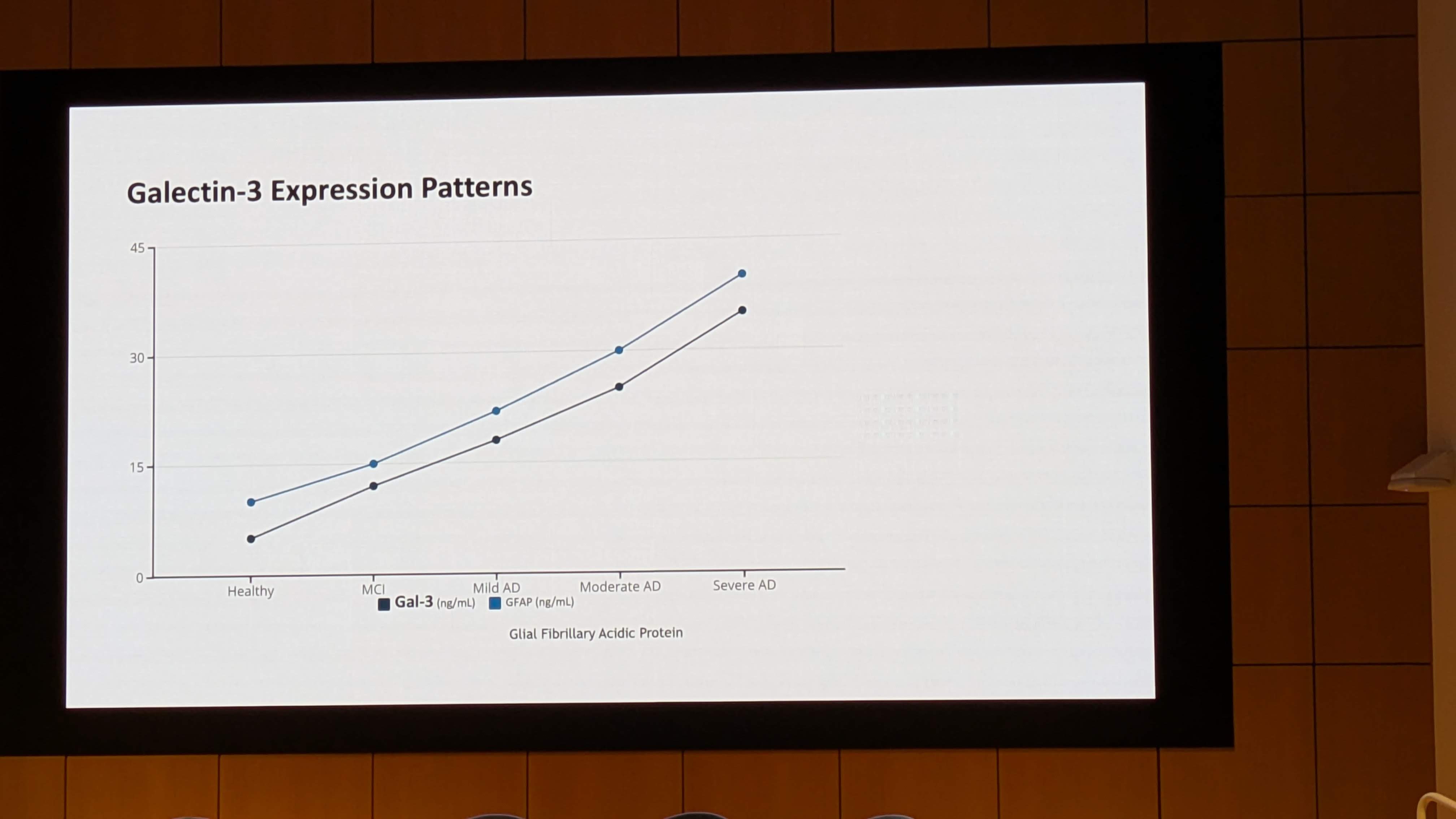
- Population: 101 patients with hypertension. Crucially, they were screened for elevated Galectin-3 levels (>13.8 ng/mL for women, >12.5 ng/mL for men). This enriched the trial for patients theoretically most likely to benefit.
- Intervention: PectaSol-C MCP vs. Placebo for 6 months.
- Primary Endpoint: Markers of collagen metabolism (serum PIIINP, PICP, TIMP-1) as proxies for cardiac fibrosis.
- Secondary Endpoint: Changes in serum Galectin-3 levels.
Results: The results were stark and largely negative regarding the “lowering” hypothesis.
- Fibrosis Markers: Treatment with MCP did not reduce markers of collagen synthesis. In fact, levels of PIIINP (a marker of collagen type III formation) showed a borderline statistically significant increase in the MCP group compared to placebo (p = 0.05).
- Galectin-3 Levels: The study did not report a significant reduction in serum Galectin-3 levels in the treatment group. The intervention failed to “move the needle” on the biomarker it targets.
- Vascular Stiffness: No change in arterial stiffness or echocardiographic parameters.
Critical Analysis: Why did this trial fail to replicate the animal success?
- Chronicity: The patients had long-standing hypertension. Established human fibrosis is highly cross-linked and resistant to degradation, unlike the nascent fibrosis in rat models.
- Dosing: The study presumably used standard dosing. It is possible that the concentration of MCP achieved in human serum was insufficient to saturate the Gal-3 lattice in the myocardium.
- Implication for the User: This study is strong evidence that MCP does not reliably lower serum Galectin-3 in patients with established cardiovascular disease. Taking PectaSol-C with the sole expectation of seeing a lower number on a LabCorp Galectin-3 test is not supported by this data(Galectin-3 Inhibition With Modified Citrus Pectin in Hypertension - PubMed).
5.2 The Prostate Cancer Trials (Keizman et al.)
In contrast to the cardiovascular data, the oncology trials paint a picture of functional success, though they remain ambiguous on the biomarker “lowering” question.
- Study Design: A multi-center, prospective Phase II study evaluating PectaSol-C in patients with Biochemically Relapsed Prostate Cancer (BRPC-M0). These are men who have had surgery/radiation but have rising PSA levels, indicating recurrence, yet have no visible metastases on scans.
- Intervention: PectaSol-C at 4.8 grams three times daily (14.4 g/total) for 6 months, with an extension to 18 months for responders.
- Primary Outcome: Prostate-Specific Antigen Doubling Time (PSADT). This measures the velocity of cancer growth. A longer doubling time means slower disease progression.
Results:
-
Efficacy: 78% of patients responded to therapy.
- PSADT: The median doubling time improved significantly, extending from a rapid growth phase to a slower, more manageable progression.
- Disease Stability: 58% of patients saw their PSA levels stabilize or decrease.
- Scan Negativity: Responders remained free of metastatic progression on scans.
- Galectin-3 Analysis: While serum Galectin-3 levels were measured as a secondary endpoint, the published reports focus heavily on the PSA dynamics. There is no explicit data in the key abstracts or summary tables demonstrating a statistically significant, population-wide reduction in serum Galectin-3. The success of the trial was defined by clinical utility (PSA slowing) rather than biomarker reduction.
Interpretation: The divergence between the Lau study (negative) and the Keizman study (positive) highlights the concept of Functional Inhibition. In prostate cancer, Galectin-3 helps circulating tumor cells clump together to survive in the bloodstream and stick to vessel walls to form metastases. By blocking the CRD, PectaSol-C prevents this adhesion. The cancer cells remain vulnerable to the immune system or simply fail to seed new sites. This benefit does not require the Galectin-3 protein to disappear; it only requires it to be blinded. The improvement in PSA kinetics is a downstream result of the cancer being functionally inhibited(Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Long-Term Results of a Prospective Phase II Study - PMC).
5.3 Safety and Tolerability
Across these trials, PectaSol-C has demonstrated a remarkable safety profile.
- Adverse Events: In the prostate cancer trial, no Grade 3 or 4 (severe) toxicities were reported over 18 months of high-dose therapy.
- Side Effects: The most common side effect is mild gastrointestinal distress (bloating, loose stools), consistent with the ingestion of carbohydrate fibers. This is generally transient.
- GRAS Status: MCP is recognized as Generally Regarded As Safe (GRAS) by the FDA, allowing its sale as a supplement.
6. The “Biohacker” Synthesis: Implications for Protocol Design
For the biohacker or clinician interpreting this data, the nuance is critical. The simplified marketing claim—“PectaSol lowers Galectin-3”—is technically unsupported by human data. However, the claim “PectaSol neutralizes the pathological effects of Galectin-3” has robust support in specific contexts.
6.1 The Disconnect: Biomarker vs. Bioactivity
The most important insight from this analysis is the decoupling of Serum Concentration from Tissue Activity.
- Serum Gal-3: Represents the total pool of the protein in circulation.
- Tissue Activity: Depends on the protein’s ability to bind ligands.
- PectaSol Effect: PectaSol floods the system with decoys. The Gal-3 binds to the PectaSol instead of the tissue. The assay used to measure Gal-3 (ELISA) likely detects the protein whether it is bound to PectaSol or not. Therefore, the lab test shows “high” Galectin-3, even though the protein is functionally inert (capped by pectin).
Actionable Advice: Clinicians should avoid using serum Galectin-3 levels as a monitoring tool for PectaSol efficacy. A patient taking 15g of PectaSol may still have a Gal-3 level of 20 ng/mL, but their risk of metastasis or fibrosis progression may be significantly reduced compared to an untreated patient with the same level. Efficacy must be measured by functional outcomes (e.g., PSA kinetics, inflammatory markers like CRP, or imaging) rather than the Gal-3 number itself.
6.2 Dosing and Protocol
The data suggests that saturation is key. The successful prostate cancer trials used 14.4 grams per day. The heavy metal study used 15 grams.
- Maintenance vs. Therapeutic: Biohackers often use 5g/day for “maintenance.” While plausible for gut health or mild chelation, the data does not confirm this is sufficient to block systemic Galectin-3 in the face of active disease. The “lock and key” model implies that you need enough keys (pectin) to fill all the locks (Gal-3).
- Timing: Given the carbohydrate nature, taking it away from meals (as done in trials) avoids potential binding with dietary proteins or minerals, though the chelation study suggests it does not rob the body of essential minerals like Calcium or Magnesium(The effect of modified citrus pectin on urinary excretion of toxic elements - PubMed).
6.3 The Conflict of Interest Factor
It is impossible to ignore the role of EcoNugenics and Dr. Isaac Eliaz in this ecosystem. Dr. Eliaz is the inventor, patent holder, and a frequent co-author on the positive studies. This does not invalidate the data—the prostate cancer trial was a multi-center study involving independent oncologists—but it necessitates a higher burden of proof. The negative result in the independent Lau et al. hypertension study stands as a significant counterweight to the company-sponsored success, suggesting that MCP is not a panacea for all Gal-3 mediated conditions.
7. Conclusions
The investigation into whether PectaSol-C lowers Galectin-3 in humans leads to a nuanced conclusion that separates functional inhibition from biomarker reduction.
- Mechanism: PectaSol-C is a Competitive Antagonist. It binds to the Carbohydrate Recognition Domain of Galectin-3, preventing the formation of the pathological lattices that drive fibrosis and cancer metastasis.
- Pharmacokinetics: Unlike native pectin, PectaSol-C (<15 kDa) is systemically absorbed, as evidenced by its ability to increase the urinary excretion of sequestered heavy metals in humans.
- Serum “Lowering”: There is no consistent evidence from human clinical trials that PectaSol-C significantly lowers the serum concentration of Galectin-3. The Lau et al. hypertension study explicitly found no reduction.
- Clinical Efficacy: Despite not lowering the serum marker, PectaSol-C demonstrates functional efficacy in blocking disease progression, most notably in biochemically relapsed prostate cancer (PSA doubling time improvement).
- Final Verdict: PectaSol-C should be viewed as a functional blocker, not a clearing agent. It neutralizes the threat of Galectin-3 without necessarily removing the protein from the blood. For the clinician and biohacker, this means relying on clinical outcomes rather than serum Galectin-3 assays to judge efficacy.
Full Gemini Deep Search Report: https://gemini.google.com/share/4bd644d9b25d
1 Like
Yes, I had seen that before. It doesn’t clear out the G3. My daughter took it for her mucinous ovarian cancer and she thought it helped, but she was doing many things. Also she tested her G3 several times and though it was always low enough it didn’t seem to go lower. So her case agrees with the study.
It looks like maybe it ties up the G3 without clearing it out? I really like that it binds heavy metals too since I believe they make heart disease so much worse. I don’t know whether plugging up the locks on the G3 somehow helps with heart disease or not. I got a bad CAC a few years ago and worry about my tubes. I take it daily.
1 Like